WHAT IS ALREADY KNOWN ON THIS TOPIC
-
Bilateral intermittent theta burst stimulation (iTBS) offers an effective and well-tolerated treatment for depression, and one that can be administered multiple times daily, as an ‘accelerated’ treatment schedule. However, some uncertainty remains over the optimal intersession interval and whether twice-daily sessions impact the speed of improvement.
WHAT THIS STUDY ADDS
-
While all groups showed an improvement in clinical outcomes, there were no group differences in terms of the pace of improvement or efficacy, among those who received bilateral dorsomedial prefrontal cortex (DMPFC)-iTBS with either a 30-min or 60-min interval between two sessions, compared with those who received the two sessions with a 0-min interval.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
-
The results suggest that twice-daily, bilateral DMPFC-iTBS at intervals of 30-min or 60-min may not accelerate the response to treatment, compared to a control interval of 0-min, when the total number of sittings and pulses per day are held constant.
Introduction
Repetitive transcranial magnetic stimulation (rTMS) is an effective, evidence-based treatment for those suffering from major depressive disorder (MDD). Numerous randomised controlled trials, large-scale naturalistic studies and meta-analyses have established the antidepressant efficacy of rTMS,1 including in those with treatment-resistant depression (TRD).2 3 Current reports have established that rTMS generally achieves response and remission rates of 50–63% and 30–35%, respectively.4–6 However, there remains considerable variability in the proportion of responders and remitters seen across studies. The variability in treatment outcomes may be due, in part, to the considerable between-studies variability in treatment parameters, including the site of stimulation, frequency, intensity, course length, number of pulses and intersession interval (ISI). Optimising these parameters may improve clinical efficacy and facilitate the widespread use of rTMS.
A standard acute rTMS treatment course includes 20–30 daily sessions, delivered over the span of 4–6 consecutive weeks, with each session lasting 19–45 minutes.7 The time commitment associated with a treatment course can be logistically challenging for patients. The newer intermittent theta burst stimulation (iTBS), directly addresses key cost and capacity concerns, given that the stimulation time is 1/6th of the time (~3 min vs 19 min) of the commonly used standard rTMS protocol.8 9 The brief nature of this protocol facilitates the administration of multiple treatments per day, in an accelerated regimen. Encouragingly, open-label and randomised studies have demonstrated the viability of accelerated rTMS, with outcomes that were similar to conventional once-daily protocols.10–13 In addition to being safe and well-tolerated, accelerated rTMS has shown the potential to rapidly and dramatically improve depressive symptoms when combined with personalised functional neuroimaging.14
While the most commonly used target for the treatment of MDD is the dorsolateral prefrontal cortex (DLPFC), evidence suggests that alternative prefrontal regions play a central role in the pathophysiology of MDD.15 One such region is the dorsomedial prefrontal cortex (DMPFC). Several case reports, open-label, and pilot studies have demonstrated proof-of-concept evidence that DMPFC-rTMS and iTBS are safe and well tolerated.16 17 However, there are currently mixed results as to the overall clinical efficacy of DMPFC-rTMS, with one recent study showing a lack of clinical difference between active treatment and sham.18 In contrast, a more recent randomised trial reported non-inferiority of rTMS delivered with the H7 coil compared to the H1 coil in reducing depression symptoms.19 The results of this trial contributed to the Food and Drug Administration clearance of the TMS protocol using the H7 coil for the treatment of depression. The H7 coil was designed to target primarily the DMPFC and cingulate cortex; however, many other structures are stimulated by the e-field from this coil. Taken together, current literature supports the potential use of DMPFC-rTMS in the treatment of MDD, yet highlights the need for further investigation.
Contrasting results in the literature also exist regarding the optimal intersession interval for TMS sessions. A recent meta-analysis of 11 accelerated rTMS studies reported ISIs ranging from 12 minutes to 2 hours.20 Research suggests that longer ISIs, of approximately 1 hour and above, induce a cumulative effect on synaptic strengthening, compared with shorter ISIs.21–24 Whether ISIs similarly impact clinical outcomes, has yet to be determined. A recent randomised trial incorporating controls for the overall number of pulses delivered to the DLPFC, daily therapeutic contact, and schedule of visits found no difference in clinical outcomes for 30 days of twice-daily treatment with 2×600 pulses of iTBS delivered over 50 minutes apart versus 0 minutes apart.11 However, an open-label study where patients received the same total number of pulses per day, either at once in a single session, or in two fractions, delivered at an 80-min interval, found that twice-daily rTMS resulted in similar treatment outcomes to once-daily rTMS yet in half the amount of time.25 Thus, some uncertainty remains over not only the optimal intersession interval for TMS, but also whether twice-daily sessions impact the speed of improvement, under rigorously controlled conditions.
As such, the overall aim of this randomised controlled study was to assess the efficacy and time course of a twice-daily bilateral iTBS protocol at two different intersession intervals (ie, at 30-min or 60-min intervals) and a once-daily bilateral iTBS protocol (ie, 0-min interval), with the number of pulses and schedule of treatment sessions held constant, in patients with TRD. We hypothesised that twice-daily bilateral iTBS at 30-min and 60-min intervals would result in a more rapid improvement on the 17-item Hamilton Rating Scale for Depression (HRSD-17) over the 20 days of treatment, compared with once-daily bilateral iTBS. As an additional analysis, we also explored the rates or response and remission across the three intersession intervals as assessed by the HRSD-1726 and the self-rated 12-item Beck Depression Inventory (BDI-II).27
Methods
Participants
The trial was registered with ClinicalTrials.gov.
From March 2018 to November 2019, we recruited adult outpatients aged 18–59 who met the following criteria: (1) had a Mini-International Neuropsychiatric Interview (MINI)28 confirmed diagnosis of MDD, single or recurrent, or bipolar disorder with a current major depressive episode, (2) had failed to achieve a clinical response to an adequate dose of an antidepressant based on an Antidepressant Treatment History Form (ATHF) score of >3 in the current episode29 30 OR had been unable to tolerate at least two separate trials of antidepressants of inadequate dose and duration (ATHF 1 or 2), (3) had a score >18 on the HRSD-17 item, (4) had no increase or initiation of any psychotropic medication in the 4 weeks prior to screening, (5) were able to adhere to the treatment schedule, (6) passed the TMS safety-screening questionnaire and (7) had normal thyroid functioning and no clinically significant abnormalities on complete blood count, on pre-study blood work.
Patients were excluded if they: (1) had a history of substance dependence or abuse within the last 3 months, (2) had a concomitant major unstable medical illness, cardiac pacemaker, or implanted medication pump, (3) had active suicidal intent, (4) were pregnant, (5) had a lifetime MINI diagnosis of schizophrenia, schizoaffective disorder, schizophreniform disorder, delusional disorder or current psychotic symptoms or (6) had a MINI diagnosis of obsessive compulsive disorder, post-traumatic stress disorder (current or within the last year), anxiety disorder (generalised anxiety disorder, social anxiety disorder, panic disorder) or dysthymia, assessed by a study investigator to be primary and causing greater impairment than MDD, (7) had a diagnosis of any personality disorder, assessed by a study investigator to be primary and causing greater impairment than MDD, (8) had failed a course of Electroconvulsive Therapy (ECT) in the current episode or previous episode, (9) had any significant neurological disorder or insult, (10) had an intracranial implant (eg, aneurysm clips, shunts, stimulators, cochlear implants or electrodes) or any other metal object within or near the head, excluding the mouth, that cannot be safely removed, (11) if participating in psychotherapy, were not in stable treatment for at least 3 months prior to entry into the study, (12) had clinically significant laboratory abnormality, in the opinion of the investigator, (13) were currently (or in the last 4 weeks) taking more than lorazepam 4 mg daily (or equivalent) or any dose of an anticonvulsant due to the potential to limit rTMS efficacy and (14) had non-correctable clinically significant sensory impairment.
Procedures
Randomisation and blinding
Participants were randomised into the study, stratified by severity of treatment resistance (ATHF<4 vs ATHF≥4). The groups were balanced with respect to ATHF scores, to control for the potential relationship between the degree of treatment resistance and rTMS response. Randomisation was based on a stratified randomisation scheme using a permuted block method with a random number generator. The block size was fixed and investigators were blinded to the randomisation block size. An independent assistant, external to the study, managed the randomisation of participants. Due to the nature of the treatments, rTMS technicians were not blinded; however, participants and all study personnel involved in the administration of clinical assessments, remained blind to treatment assignment.
Study design
Resting motor threshold (RMT) and coil position were determined using previously described methods for bilateral DMPFC-rTMS.17 25 rTMS treatments were administered using the MagPro R30 or X100 rTMS device (MagVenture, Farum, Denmark) with a Cool-DB80 coil. All participants received bilateral DMPFC-iTBS (triplet bursts at 50 Hz, repeated at 5 Hz), delivered with a duty cycle of 2 s on, and 8 s off, for a total of 600 pulses per hemisphere (1200 pulses total), twice per treatment day (2400 pulses). The coil vertex was positioned over the DMPFC at a position 25% of the distance posteriorly along the midline from nasion to inion, as described in previous reports from our group.17 Stimulation was delivered at 120% of the RMT.
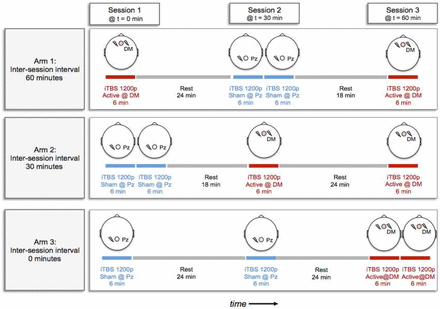
Participants received two sessions per day of bilateral DMPFC-iTBS at 60-min, 30-min or 0-min intervals, on weekdays, for a total of 20 days of treatment (ie, 40 active sessions in each group). Refer to figure 1 for a schematic of the experimental design. Sham treatments were delivered using a shielded ‘sham coil’ that produced the auditory and tactile sensations of stimulation. As in previous work by our group,11 sham treatments were delivered to a different scalp location (Pz) than active treatments, as the sensations have been shown to be distinguishable when delivered to the same site in the same patient. The incorporation of sham rTMS over Pz was intended to control for non-specific therapeutic-contact effects, such that all patients sat for three sessions of stimulation (at 0, 30 and 60 min) on each treatment day (figure 1). To further ensure blinding, participants were told that the aim of the trial was to investigate the stimulation effects across two brain regions over three sessions. Additionally, they were told that the stimulation sensation would feel different for the two regions.


Schematic of the experimental design showing daily treatment protocols for 60 min, 30 min and 0 min interval treatment arms. DM, dorsomedial; iTBS, intermittent theta burst stimulation; Pz, scalp location.
Outcome measures
Clinical outcome measures were assessed at baseline, weekly over the 4-week treatment course (5 days/week on weekdays) and during follow-up visits at 1 week, 4 weeks and 12 weeks post-treatment. The HRSD-17 was the primary outcome measure. The BDI-II27 was included as a secondary outcome measure.
Statistical analysis
For the primary hypothesis, a mixed-effects linear regression model was used to assess for differences in the pace of improvement over time. Group, time, and group-by-time interaction were used as explanatory variables, with participant as a random factor. For the additional analyses, the response was defined as ≥50% reduction in symptoms on the primary and secondary outcome measures (ie, HRSD-17 and BDI-II). Remission was defined as a score of ≤7 for the HRSD-17 and a score of ≤12 for the BDI-II at the final treatment visit. Response and remission rates were calculated based on the intent-to-treat (ITT) samples. Fisher’s exact test was employed to assess the significance of any observed differences in the proportion of responders and remitters between groups. Given that DMPFC modulation has been suggested to be more effective for specific depressive symptom domains,16 we repeated our main primary analysis with anhedonia as the variable of interest. Anhedonia was assessed from a subset of items on the BDI-II (ie, items 4—loss of satisfaction/enjoyment, 12—loss of interest and 21—loss of interest in sex).31
Results
Of the 245 participants assessed for eligibility, 63 participants were ineligible or declined to participate (see figure 2). 182 participants were randomly assigned to receive rTMS (0-min ISI: n=60; 30-min ISI: n=63; 60-min ISI: n=59); 4 of these participants were randomised but did not receive treatment. Of the remaining 178, 20 participants withdrew from the study before completing a full course of treatment (0-min ISI: n=6; 30-min ISI: n=5; 60-min ISI: n=9). Demographic and clinical characteristics for each of the three groups are presented in table 1. Of note, 7 participants were not included in the secondary HRSD-17 analyses of response and remission due to missing baseline and/or final treatment scores, likewise, 17 participants were not included due to missing BDI-II assessments.
Baseline demographic and clinical characteristics for all participants who completed 20 days of treatment


Consolidated Standards of Reporting Trials diagram illustrating patient flow in the present study. ISI, intersession interval.
Clinical outcomes
Contrary to our hypothesis, a linear mixed-effects model revealed no significant difference in the pace of improvement between groups over time (group×time interaction, HRSD-17, F=0.75, p=0.72; BDI-II, F=0.68, p=0.79). There was however, a significant overall main effect for time (HRSD-17, F=35.65, p<0.01; BDI-II, F=32.15, p<0.01), but not for the group (HRSD-17, F=0.40, p=0.67; BDI-II, F=1.53, p=0.22) (see figure 3). Visual inspection of residual plots did not reveal any obvious deviations from homoscedasticity or normality. Similarly, our exploratory analysis of anhedonia revealed no significant group×time difference (F=1.13, p=0.34). There was a significant overall main effect for time (F=54.35, p<0.01), but not for group (F=0.79, p=0.45).


Change in symptom severity over the course of treatment in patients receiving DMPFC-iTBS either at a 0-min ISI, 30-min ISI or 60-min ISI on the HRSD-17 and the BDI-II scales. Error bars represent the SD of the mean for the sample. Figures represent change in total scores on the HRSD-17 (A) and BDI-II (B), as well as percentage change over time on the HRSD-17 (C) and BDI-II (D). BDI-II, Beck Depression Inventory; DMPFC, dorsomedial prefrontal cortex; FU, follow-up; HRSD-17, 17-item Hamilton Rating Scale for Depression; ISI, intersession interval; iTBS, intermittent theta burst stimulation.
The ITT response rates, as assessed by the HRSD-17, for the 0-min, 30-min and 60-min groups were 27.8% (n=15), 35.7% (n=20) and 18.8% (n=9), respectively (Fisher’s exact test p=0.16). Similarly, the ITT remission rates did not differ between the groups; the remission rates for the 0-min, 30-min and 60-min groups were 11.1% (n=6), 19.6% (n=11) and 10.4% (n=5) (Fisher’s exact test p=0.31). Response and remission rates also did not differ between the groups as assessed by the BDI-II (refer to table 2 for detailed results).
Clinical outcomes for the 0, 30 and 60 min ISI study arms
Likewise, continuous measures revealed no significant between-group differences. For the HRSD-17, endpoint scores in the 0-min, 30-min and 60-min groups were 14.5±6.2, 14.2±6.6 and 15.1±6.5, respectively (F(2,148)=0.54, p=0.58). The mean percentage of improvement from baseline to day 20 of treatment on the HRSD-17 also revealed no significant group differences (F(2,148)=0.61, p=0.54). Similarly, no significant differences were found between groups on endpoint BDI-II scores (F(2,138)=2.0, p=0.13) or the mean percentage of improvement from baseline to day 20 of treatment on the BDI-II (F(2,138)=1.11, p=0.33).
Of note, a post hoc power analysis was performed using G*Power (www.gpower.hhu.de), setting the significance at 0.05 and the power at 80%. Results from the power analysis indicated that the study had moderate statistical power (critical F=3.06, df=150, power=0.366, effect size f=0.120), leaving open the possibility of Type II errors (ie, final outcomes may have significantly differed between groups had we tested on a larger patient sample).
Safety and tolerability
The safety profile was benign across all groups; no seizures or other serious treatment-limiting adverse events occurred in any of the patients. A Fisher’s exact test revealed no significant difference in the number of withdrawals, across the three treatment groups (two-tailed p=0.34). Of note, 15/158 participants (0-min ISI: n=6; 30-min ISI: n=5; 60-min ISI: n=4) did not reach the stimulation threshold of 120% of RMT due to intolerability. Of the 15 participants, 8 were considered treatment responders (≥50% reduction in HRSD-17 scores). The average day that the maximum stimulation intensity was reached was day 9, for both the left and right sides. It should be noted that the average pain score of 6 out of 10, is higher than that of standard DLPFC stimulation.8 11 Refer to table 3 for the adverse events and pain scores across the treatment groups.
Adverse events and pain score
Discussion
Accelerated rTMS regimens, consisting of multiple daily sessions, are becoming increasingly common in both research and clinical settings. The optimal intersession interval for such regimens, however, has received relatively little systematic study in the literature to date. As such, the aim of the present study was to investigate the optimal ISI for accelerated DMPFC-iTBS, comparing 60-min, 30-min and 0-min treatment intervals. Concerning overall clinical improvement, no significant differences between the groups were observed on the HRSD-17 or the BDI-II. Likewise, no significant differences were found between the groups on rates of response or remission. However, all three iTBS protocols led to lower-than-expected reductions in depressive symptoms and lower response and remission rates than seen in a similar DLPFC study. Overall, these findings suggest that, contrary to our original hypothesis, implementing a 30-min or 60-min interval between two sessions of DMPFC-iTBS did not lead to faster outcomes, compared with once-daily iTBS.
Though not a key outcome of this study, the response (ie, 20–30%) and remission (ie, 10–20%) rates were lower than conventional rTMS treatment trials targeting the DLPFC, which report response and remission rates of approximately 50% and 30%, respectively.32 33 One recent sham-controlled study found that 30 twice-daily sessions of active DMPFC rTMS did not result in improvements in depressive symptoms greater than that of sham stimulation, in 120 patients with TRD.18 A similar lack of difference was found in an earlier treatment study, between mediofrontal double cone coil rTMS and sham stimulation, in post hoc analyses.34 Taken together, this suggests that the DMPFC may be an inferior therapeutic target compared with the DLPFC; further double-blind studies investigating the clinical efficacy of DMPFC-rTMS may be warranted. Additionally, future work using DMPFC stimulation should include a validated anhedonia rating scale, as stimulation of this region may target these symptom domains more specifically than DLPFC stimulation.
Regarding the pace of improvement, our findings are in line with results from a recent, large multisite randomised controlled trial of accelerated DLPFC-rTMS in MDD11. In this study no differences in post-treatment outcomes or trajectories of improvement were seen for twice-daily sessions of DLPFC-iTBS spaced by a 0-min versus a 50-min interval. These findings, along with the current study would suggest that the number of treatment sessions may not be linearly related to the rate of recovery. However, contrasting findings regarding the pace of clinical improvement have been reported.25 35 It has been proposed that the total number of daily sessions rather than the total number of daily pulses may play a more significant role in facilitating an accelerated response to treatment.25 36 37
It is also important to acknowledge the discrepancy between the findings of a lack of acceleration seen in the present study versus the findings of robust acceleration in studies using 10× daily left DLPFC-iTBS with a 50-minute interval.14 38 Important differences exist between these protocols and the protocol of the present study; these include a much higher number of sessions per day (10 vs 2), a slightly higher number of total sessions (50 vs 40), a different number of pulses per session/site (1800 iTBS vs 600 iTBS in the present study), a different intensity (90% RMT adjusted based on MRI vs 120% RMT in the present study) and a different target (Functional magnetic resonance imaging (fMRI)-personalised left DLPFC, vs non-personalised DMPFC in the present study). Given the discrepancy in study outcomes, it is clear that further research is required to better understand the potential impact of treatment parameters such as sessions/day, sessions/course, pulses/session, stimulation intensity and stimulation target, on symptom improvement.
Previous neurophysiological evidence has suggested that rTMS can induce cumulative plastic changes of intrinsic motor cortex excitability with repeated, spaced application.36 37 However, this earlier work involved three sessions of rTMS applied to the primary motor cortex in healthy participants over the course of 1 day. In the preclinical literature, ISIs ranging from 50 to 90 minutes have been associated with synaptic strengthening, while ISIs of less than 40 minutes have failed to achieve the same effect.21–24 The distinct time courses of effects between the temporary 50-to-90-minute period of motor evoked potential (MEP) facilitation versus the 20-to-30-day time course for DMPFC-rTMS as indexed by improvement in depressive symptoms, may indicate fundamentally different mechanisms of effect—such as ‘late’ long-term potentiation over timescales of weeks to months.39 Additionally, more recent clinical controlled trials have moved toward more intensive protocols including 5–10 treatment sessions per day. It is possible that the length of ISIs may have a larger impact on clinical outcomes in these condensed treatment courses.
Importantly, all stimulation protocols were shown to be safe. However, there were some notable tolerability issues, given that 15 participants were unable to reach the maximum stimulation intensity (ie, 120% of RMT) and the average day that the maximum intensity was reached was day 9. While this study did not directly compare DMPFC to standard DLPFC stimulation, the reported average pain score of 6 out of 10 was higher than those generally reported in the literature, using similar pain scales.8 11 It is possible that the bilateral stimulation protocol and coil orientation contributed to the heightened pain scores and therefore alternative coil orientations may be more tolerable. It is also unclear to what extent the tolerability issues contributed to the lower-than-expected response and remission rates.
The present findings should be interpreted in light of several limitations. An important consideration when interpreting the present findings is the possibility of expectancy effects. All patients were informed they would receive accelerated rTMS, which could account for the rapid improvement seen in patients receiving more densely scheduled sessions (0-min ISI and 30-min ISI). Additionally, rapid treatment response has previously been observed in some individuals with sham accelerated TMS,40 and the amplification of this effect through the use of more intensive protocols is possible. While participants were blinded to the pattern of stimulation across two brain regions, they were not aware that one stimulation site would deliver ‘sham’ treatments. As such, we did not collect information on the integrity of the blinding, as asking participants would unblind them. An additional limitation of the design is that we did not collect rater guesses with respect to group allocation. Finally, this study was moderately powered, thereby leaving open the possibility of Type II errors.
In conclusion, the results of the present study of DMPFC-rTMS are consistent with the results of the previous DLPFC-rTMS11 finding that twice-daily stimulation at intervals of 30 or 60 minutes does not appear to accelerate the response to treatment, compared with a control interval of 0 minutes, when sham control sessions are added to match the total number of sittings and pulses per day. In spite of the negative findings of the current study, certain accelerated TMS regimens nonetheless appear to offer a major potential advancement in the treatment of depression. The robust and rapid improvement seen in recent trials of highly accelerated regimens14 41 indicates that acceleration of treatment response may be achievable with certain sets of treatment parameters, if not others. Further study will be required to determine whether acceleration may ensue with specific factors such as more sessions per day, more pulses per session, more personalised targeting, or with non-specific factors such as patient expectancy. In addition, the impact of expectation and placebo on short and long term outcomes with accelerated treatments relative to once-daily treatments has been relatively unexplored. Given the major benefits that may ensue if TMS response can be reliably achieved in days rather than weeks, such work is likely to offer substantial translational impact.
Data availability statement
Data are available upon reasonable request. Data may be made available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
The study was conducted at two Canadian academic health sciences centres (Centre for Addiction and Mental Health and University Health Network) with approval from their research ethics boards (ID-002/2018). Participants gave informed consent to participate in the study before taking part.

![[Watch] Exploring Possibilities of Investing Now for Prosperous, Sustainable Neighborhoods](https://fitandvibrantlife.com/wp-content/uploads/2024/10/connecting-communities-exploring-possibilities-investing-prosperous-sustainable-neighborhoods-1068x712.jpg)