Introduction
Psychosis, as an umbrella term, covers a diverse range of psychiatric conditions, such as schizophrenia, schizoaffective and other disorders, which share overlapping features of neurobiology and genetic liability across subtypes.1–3 Recent neuroscientific and brain imaging developments and progress in understanding the striatal structure, a key component of the corticobasal ganglia circuitry, have further enhanced our insights on the neurobiological underpinnings of psychotic symptoms. In fact, the latest neuroimaging data appear to challenge the traditional mesolimbic theory and imply that dopaminergic dysfunction in schizophrenia is most prominent within the dorsal striatum, as opposed to the ventral regions of the striatum.4 Moreover, a recent large-scale study analysing available imaging data from research groups worldwide demonstrated subtle structural asymmetries in the basal ganglia structures, specifically the pallidum, which is more pronounced in older adults with schizophrenia compared with healthy controls.5 An altered asymmetry and other changes in basal ganglia volumes found in first-episode patients further stipulate their potential involvement in the pathophysiology of psychosis.6
Clinically, dysfunction in the basal ganglia, specifically the dorsolateral portion subserving for sensorimotor functions, has been linked to several movement disorders,7 including dyskinesia, dystonia, parkinsonism and akathisia. Dyskinesia is typically described as hyperkinetic, varying in intensity choreiform involuntary movements. Dystonia manifests in sustained or intermittent involuntary muscle contractions, such as twisting and repetitive movements and posture. Parkinsonism is clinically defined by core features of tremor, rigidity, bradykinesia and postural instability.8 Akathisia is typically characterised by both a subjective feeling of inner restlessness and objective semi-purposeful, complex motor activities, such as pacing around.9 These abnormal involuntary movements are commonly associated, but not exclusively limited, to medication use, particularly antipsychotics in patients with schizophrenia. Intriguingly, reports of involuntary abnormal movements in individuals with schizophrenia predate the introduction of antipsychotic medications, which points to the likely occasional, spontaneous origin of such disturbances.10
A growing body of evidence suggests potential shared neural mechanisms implicated in both motor functions and the occurrence of psychosis. A first narrative synthesis on spontaneous movement disorders (SMDs) in never-treated first-episode patients, conducted by Pappa and Dazzan,11 found a median prevalence of 9% for dyskinesia and 17% for parkinsonism. In addition, subsequent studies examining ultra-high-risk individuals proposed that abnormal movements can be present before and even predict the future conversion to psychosis.12 13 Furthermore, a separate meta-analysis14 revealed a significantly higher prevalence of dyskinesia and parkinsonism in first-degree relatives of individuals with schizophrenia compared with healthy controls. Therefore, it is possible that there may be a genetic susceptibility for disease development, especially in the context of pre-existing movement disorders within the family.
Age-associated increases in prevalence rates of drug-induced movement disorders have been well documented in the literature observing older populations.15 16 Similarly, there is evidence supporting differences related to other pertinent factors, such as sex and ethnicity,17 18 which may be linked to genetic predisposition.19 On the other hand, a more comprehensive understanding of specific risk factors and characteristics of SMDs across psychotic disorders is lacking. Therefore, the aim of this systematic review and meta-analysis is to evaluate and compare the prevalence rates of SMDs in antipsychotic-naïve individuals with chronic psychosis and first-episode psychosis (FEP). In addition, we aim to gain a more nuanced understanding of the factors that may be influencing the prevalence of SMDs.
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.20 The study was registered with PROSPERO (registration number: CRD42024501951).
Search strategy and eligibility criteria
The literature search for this review was conducted via Ovid and included three main databases: MEDLINE, Embase and PsycINFO. The final search was completed in May 2024. The search was narrowed by applying an English language filter and specifying the inclusion of studies with human subjects only. Additionally, preprints and studies with no available abstract were excluded from consideration. Finally, a search of grey literature was conducted using the relevant key words, however no additional findings were identified.
Studies were deemed eligible for inclusion if they: (1) included patients experiencing their first episode of psychosis or those with a chronic psychotic illness, (2) included patients with no history of treatment with antipsychotic medication and (3) were reporting on the presence of SMDs using validated clinical assessment tools.
Exclusions were applied to reviews, conference abstracts, book chapters, protocols, editorials, opinion pieces, discussions, commentaries, case reviews or series as well as studies not in the English language.
Screening and data extraction
To facilitate the study screening process, the search results were transferred to an online software tool, Covidence. Two reviewers, AK and KJ, independently screened the titles and abstracts of studies obtained through the search strategy to identify those meeting the predefined inclusion criteria. The full text of eligible studies was acquired and independently assessed for suitability by the same two reviewers. Any disagreements in eligibility assessments were resolved through discussion involving a third reviewer, SP.
Data from each study was independently extracted into an Excel spreadsheet by two reviewers (AK and KJ), recording the following information: author, year of publication, country, study design, aim, setting, main patient demographic characteristics, inclusion/exclusion criteria, diagnosis, duration of untreated psychosis (DUP), diagnostic testing type, threshold for case definition and the prevalence of SMDs. If age and DUP were only recorded for individual groups, we calculated a weighted mean, taking into account the size of each group as the respective weights. The initial 10% of the results were compared to ensure consistency between the two reviewers, demonstrating satisfactory agreement. Subsequently, we proceeded to extract the remaining information independently.
Risk of bias assessment
The evaluation of the risk of bias was conducted independently by two reviewers, AK and KJ, using Joanna Briggs Institute (JBI) Critical Appraisal Checklists for quantitative analytical studies, allowing for responses of ‘yes’, ‘no’ or ‘unclear’.21 Depending on the study design, we used three different JBI screening tools tailored for analytical cross-sectional studies, case-control studies and cohort studies.22 Randomised controlled trials (RCTs) and open-label studies were critically appraised using a cross-sectional study checklist, as it was deemed more appropriate for addressing our specific research question on point prevalence. Questions irrelevant to the overall outcome were marked as N/A (not applicable). Individual risk of bias ratings were assigned based on the following criteria: a low risk if 70% of answers scored ‘yes’, a moderate risk if 50%–69% of questions scored ‘yes’ and a high risk if ‘yes’ scores were below 50%.23 Any disagreements between the reviewers were resolved through consensus, involving a third reviewer if needed. A full summary presenting the ratings for each domain is available in online supplemental materials.
Data analysis
We used R programming software (RStudio/2023.09.1+494) for the quantitative analysis. CIs for individual study results calculated using Clopper-Pearson exact binomial interval.24 Cochran’s Q-test and I² statistics was employed to assess potential sources of heterogeneity across studies, with I2 >75% indicating a high heterogeneity.25 As predicted, a significant between-study heterogeneity was observed, resulting in the use of a random-effects model to pool effect sizes. To stabilise variance and mitigate the issue of disproportionately inflated weights for studies with prevalence rates approaching 0, we applied a Freeman-Tukey double arcsine transformation for all the computations.26 The restricted maximum likelihood estimator27 was used to calculate the between-study variance τ2. The prediction interval was calculated using Kenward-Roger method.28
Publication bias and sensitivity analysis
We assessed publication bias using the DOI plot and Luis Furuya-Kanamori (LFK) asymmetry index,29 which quantitatively evaluates asymmetry: scores within ±1 indicate ‘no asymmetry’, scores exceeding ±1 but within ±2 indicate ‘minor asymmetry’ and, finally, scores exceeding ±2 indicate ‘major asymmetry’. In addition, the regression test by Peters et al
30 was used to explore the association between SE and effect size across the studies.
Where applicable, we employed different outlying and influential study diagnostic methods31 32 to identify studies contributing to overall heterogeneity. We conducted a subgroup analysis (assuming common τ2 across subgroups),33 based on the following moderating variables: risk of bias, diagnostic tool choice and chronic versus first-episode patients. In addition, a weighted linear regression model was used to observe the influence of a hypothesised moderator (age and DUP), where the transformed proportions were regressed against the moderator. We used a Wald-type test, using the Z-score, to assess the statistical significance of the regression model slope and to determine if the subgroups displayed significant differences in their overall effect sizes. We used the R2 index to estimate the proportion of total heterogeneity accounted for by the moderating variable.34
Results
Search results and study characteristics
Our initial search yielded a total of 711 publications. Following the de-duplication and screening, 53 studies underwent a full-text review. Among them, 27 articles met the criteria for inclusion and were subjected to quantitative analysis. A PRISMA diagram35 detailing a full study retrieval process is shown in online supplemental figure 1.
From 27 studies36–62 included in this meta-analysis (key findings are shown in table 1 and full descriptive characteristics displayed in online supplemental table 1), the majority were cross-sectional37 38 40 42 43 47 50–52 54–56 58 59 (n=14), followed by 8 cohort studies,41 44–46 49 57 60 61 2 case-control studies,36 39 2 RCTs48 62 and 1 open-label study.53 Collectively, it presented prevalence data on 2666 patients. Notably, one study49 reported prevalence estimates for two distinct cohorts; therefore, we opted to report their findings separately.
Studies reporting on spontaneous movement disorders
Of the included studies, 18 (67%) were deemed to have a moderate risk of bias,36–38 41 42 45 47 48 50–53 55 57–60 62 while 9 (33%) were at high risk of bias39 40 43 44 46 49 54 56 61 (online supplemental table 2). 17 studies38 41–45 47–49 51–54 57 58 61 62 reported data on FEP patients, and 10 studies36 37 39 40 46 50 55 56 59 60 focused on chronic patients; however, 3 studies36 37 50 in the chronic psychosis group included mixed cohorts of both patients with chronic psychosis and FEP. The mean age in the chronic patient group (n=10) was 38.9 years, whereas in the FEP group, it was 26 years (n=13; other studies only provided a range). For DUP, the mean duration was 8.3 years in the chronic patient group (n=9) and 2 years in the FEP group (n=12).
The diagnostic tools used to identify different SMDs varied across the included studies. For example, Extrapyramidal Symptom Rating Scale (ESRS),63 Abnormal Involuntary Movement Scale (AIMS)64 with or without Schooler & Kane (S&K) criteria and St. Hans Rating Scale for extrapyramidal symptoms (SHRS)65 were used to assess dyskinesia; Simpson-Angus Scale for extrapyramidal side effects (SAS),66 SHRS, Modified Rogers Scale (MRS)67 and ESRS were used to assess spontaneous parkinsonism (SP); Barnes Akathisia Rating Scale (BARS),68 MRS and ESRS were used for akathisia and ESRS was used for dystonia.
Spontaneous dyskinesia
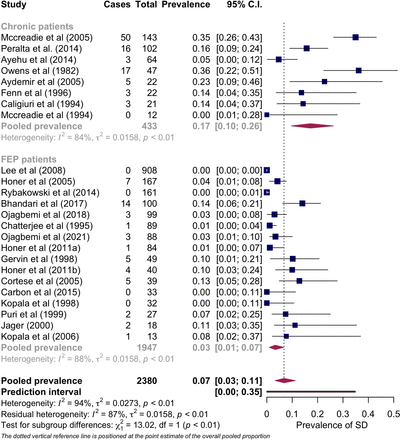
A total of 24 samples from 23 studies,36–39 41 42 44 46–58 60–62 involving 2380 patients, reported prevalence data on spontaneous dyskinesia (SD). The overall random-effects pooled prevalence of SD was 7% (95% CI 3 to 11) with substantial heterogeneity (I2=94%, p<0.01). The prediction interval (a measure indicating the expected range of future estimates)69 was between 0% and 35%, which suggests that the prevalence of dyskinesia in potential future studies may fall within this range.
In a subgroup analysis comparing patients with chronic psychosis36 37 39 46 50 55 56 60 and FEP,38 41 42 44 47–49 51–54 57 58 61 62 we observed significant differences in prevalence rates (p<0.01), which explains a substantial portion of true heterogeneity (R2
=42%). The forest plot (figure 1) illustrates a prevalence estimate of 17% (95% CI 10 to 26) and 3% (95% CI 1 to 7) in patients with chronic psychosis and FEP, respectively; however, it is noteworthy that there remained a significant unexplained residual heterogeneity (I2=87%, p<0.01).


Pooled prevalence of spontaneous dyskinesia (SD) in patients with chronic psychosis versus first-episode psychosis (FEP).
Further subgroup comparisons, stratified by the risk of bias (p=0.19) and choice of diagnostic tool for dyskinesia (AIMS using S&K criteria36–38 41 47 50 51 54–56 60 61 vs AIMS without S&K criteria39 46 49 or other rating scales42 44 48 49 52 53 57 58 62) (p=0.35) did not reveal significant differences in prevalence rates, suggesting that these factors were not significant moderating variables. The results of pooled prevalence estimates for subgroups and findings from meta-regression analysis are shown in online supplemental table 3.
Finally, a meta-regression analysis, based on available (n=18) continuous variables, showed a significant positive correlation between age36–39 41 42 44 46 47 50–56 60–62 (p<0.05) and DUP36 37 39 42 46 48–52 54–58 60 61 (p<0.05) and dyskinesia prevalence, as illustrated in scatter plots presented in online supplemental figures 2and 3. We were unable to check for possible intercorrelation between DUP and age due to reporting inconsistencies in some included studies, where one or the other variable was missing, rendering direct comparison impossible.
Spontaneous parkinsonism
The meta-analysis of SP included 21 prevalence estimates from 20 studies,37 40–45 48–53 56–62 involving a total of 1707 participants. We found that the overall pooled prevalence of SP was 15% (95% CI 12 to 20). Similar to the findings for SD, there was a high degree of heterogeneity (I2=81%, p<0.01) with a prediction interval of 2% to 37%.
As shown in figure 2, the prevalence of SP was 14% (95% CI 10 to 19) among first-episode patients41–45 48 49 51–53 57 58 61 62 and 19% (95% CI 12 to 28) among chronic patients37 40 50 56 59 60; however, these differences were not significant (p=0.27). Similarly, we did not observe any significant differences in prevalence estimates between the groups, stratified by risk of bias (p=0.77), as detailed in online supplemental table 4. Finally, a meta-regression analysis of the available continuous data, specifically age37 40–45 50 51 53 56 59–62 (n=15, p=0.09) and DUP37 40 42 43 45 48–52 56–58 60 61 (n=16, p=0.58), did not show a statistically significant correlation between these variables and the prevalence of SP, as demonstrated in scatters plots available in online supplemental figures 4 and 5.


Pooled prevalence of spontaneous parkinsonism (SP) in patients with chronic psychosis versus FEP.
Akathisia
In total, nine studies48 51 53 57–62 reported prevalence data on akathisia. However, after using outlying and influential study diagnostics (online supplemental figure 6), we excluded one study62 from the meta-analysis due to its significant influence on overall heterogeneity. Compared with other studies, it reports a considerably higher prevalence (23%) due to the use of a very low threshold for akathisia case definition. Specifically, it used a threshold of 1 (‘borderline case’) or higher on the SHRS for extrapyramidal symptoms.65 Subsequently, the pooled akathisia prevalence from the remaining 8 studies,48 51 53 57–61 involving 714 patients, was 4% (95% CI 3 to 6) using both common and random-effects methods (figure 3). Notably, the I2 statistic was 0% (p=0.65), accompanied by a 95% CI ranging from 0% to 68%, which indicates an uncertain degree of heterogeneity. Finally, the prediction interval, ranging between 1% and 9%, indicates that the future studies may expect to find spontaneous akathisia prevalence within this range.


Pooled prevalence of akathisia.
A subgroup analysis was not conducted due to the small number of studies included. However, two studies59 60 reported prevalence of akathisia for chronic patients (7% and 4%). In addition, one study61 was deemed at high risk of bias with the reported prevalence of 0%.
Dystonia
In total, only five studies49 52 53 57 58 reported the prevalence of dystonia in first-episode patients, therefore an overall result pooling was not conducted. All five studies assessed dystonia using ESRS.63 Notably, the data were highly heterogeneous, with three studies53 57 58 reporting a prevalence of 0%, and the other two studies49 52 reporting prevalence rates of 15% and 16%, with the mean prevalence of 6% (median 0%). One study49 was deemed to be at high risk of bias, while other four were categorised as being at moderate risk of bias.
Discussion
To our knowledge, this is the first meta-analysis reporting on the prevalence of SMDs in never-treated patients with both chronic psychosis and FEP. The overall pooled prevalence of SD was 7%, with 17% observed in chronic patients and 3% in the FEP group. Interestingly, the latter finding is lower than the median prevalence of 9% previously reported in a systematic review by Pappa and Dazzan.11 Regarding SP, we noted only a slightly lower prevalence estimate of 14% in the FEP group compared with reported median prevalence of 17% by Pappa and Dazzan. Additionally, we found that the SP prevalence among chronic patients was 19%, contributing to an overall pooled prevalence of 15% across both groups.
Interestingly, if we compare our results with a recent meta-analysis by Ali et al
70
,70 on the prevalence of antipsychotic-induced extrapyramidal side effects (EPSEs), we find largely similar results. The authors reported prevalence rates for antipsychotic-induced parkinsonism, akathisia and tardive dyskinesia to be 20% (95% CI 11% to 28%), 11% (95% CI 6% to 17%) and 7% (95% CI 4% to 9%), respectively, which are only slightly higher than our findings.
Consistent with the results of meta-analysis by Koning et al,14 our study found a positive correlation between both age and DUP with prevalence rates of dyskinesia, but not parkinsonism. This finding could potentially explain the significant differences observed in subgroup comparisons between the patients with chronic psychosis and FEP in the SD data compared with the SP group, where differences were not significant. In addition, Koning et al reported a significant intercorrelation between age and DUP; however, they found no significant differences regarding age between patients with schizophrenia and healthy controls, which supports the existing evidence that the risk of developing SD increases with age.71
The pooled prevalence of akathisia was 4%, based on eight out of nine studies included in this meta-analysis. Despite the relatively consistent results and low between-study heterogeneity, the findings should be viewed cautiously due to akathisia’s non-specific features and possible difficulties in differentiating restlessness specific to akathisia from, for example, psychotic agitation, or other conditions such as anxiety and substance misuse or withdrawal; hence, it has been previously argued that it may not represent a true form of spontaneous akathisia.11 For dystonia, the data were sparse and highly heterogeneous, with a mean prevalence of 6%, but a median of 0%, based on five studies (range 0%–16%). Both akathisia and dystonia were significantly under-reported compared with parkinsonism and dyskinesia, indicating a need for further research. The lower frequency and study of akathisia and dystonia possibly suggest they may be less clinically significant. Consequently, the focus of the following discussion and conclusions will be on SD and SP.
Sensitivity analysis
To address the high degree of heterogeneity, particularly in SD and SP data, we used clustering and outlying study diagnostic algorithms to identify and remove potential outliers.
Four studies46 54 56 62 in the SD data and four studies40 43 48 49 in the SP data showed outlying effect sizes, meaning that their CIs did not overlap with the CI of the pooled estimate. We also excluded three experimental design studies (two RCTs48 62 and one open-label study53) due to possible selection bias differences compared with observational studies included in this review. Subsequently, as demonstrated in table 2, the overall pooled prevalence of SD decreased to 6%, whereas for SP, it increased to 16%.
Results of sensitivity analysis for SD and SP data
We also performed influential study diagnostics to identify studies with a large impact on the pooled effect or heterogeneity. This additional analysis identified no studies as influential in the dyskinesia data and only one study43 in the parkinsonism data. Removing it did not result in any significant changes in the overall pooled prevalence, either individually or in combination with identified outliers, as demonstrated in table 2. However, it is noteworthy that both the I2 and prediction intervals decreased, particularly in the SP data, but remained substantial in SD data. Finally, supporting the findings of high heterogeneity, we observed the presence of several distinct clusters within each dataset, indicating the existence of more than one ‘population’ within the pooled data, warranting subgroup comparisons and further exploration of possible causes for this.
Potential sources of heterogeneity and methodological limitations
Due to the high between-study heterogeneity observed, particularly in SD and SP data, it is crucial to interpret the pooled prevalence estimates with caution. A subgroup analysis, stratified by the duration of illness (chronic psychosis vs FEP), revealed significant differences in SD data but not SP. Other subgroup comparisons by risk of bias and choice of diagnostic tool did not yield statistical significance, indicating that they may not be significant modifying variables in either SD or SP data. However, it is worth noting that substantial between-study heterogeneity within individual subgroups makes it more challenging to detect significant differences even if they do exist.72
With regard to risk of bias assessment, it inevitably involves a degree of subjectivity, particularly when using different tools for various study designs, which could have affected the reliability of discriminating between moderate and high risk of bias. Nevertheless, none of the included studies were classified as being at low risk, which indicated significant flaws in their methodology.
One of the primary methodological limitations were around poor sampling methods, resulting in non-representative populations, as included studies mainly relied on convenience sampling and did not conduct sample size calculations. Subsequently, there was a significant over-representation of males,37 39 40 44–46 48–54 57 potentially compromising the internal validity of the reported findings, as sex differences exist in both spontaneous and drug-related movement disorders.18 19 In addition, there were several confounding factors that were not addressed through participant selection processes, such as excluding patients with lifetime exposure to antipsychotics39 41 42 57 or considering the history of other medications and/or substance misuse.37 39 43 46 47 54–56 61
It is important to mention a considerable diagnostic variability across the included studies, with some focusing solely on schizophrenia spectrum disorders,36–39 42 44 46–48 50 52 53 55–58 60–62 while others incorporating a broader range of non-organic psychoses,40 41 43 45 49 51 54 59 including affective psychotic disorders. One study43 found a significantly higher prevalence of parkinsonism in patients with affective psychosis and schizoaffective disorder compared with those with non-affective psychoses, whereas other studies did not explore such differences.
In addition, another potential source of between-study heterogeneity was around DUP and racial diversity. The DUP varied considerably across included studies, in some cases making it challenging to differentiate between chronic and first-episode patients.36–38 42 50 52 Therefore, studies were assigned to chronic and FEP groups based on the study authors’ explicit indication that their participants included first-episode patients, which mostly corresponded with relatively lower age and shorter DUPs. On the other hand, the operational definitions and justification for such distinctions were mostly lacking, thus significantly contributing to the heterogeneity of the studies.
Regarding differences in racial or study origins, there was insufficient data to conduct a subgroup analysis, as the majority of studies did not provide data on the racial composition of their participants. Nonetheless, some significant findings emerged at the individual study level. For example, SMD rates were higher in some studies50 56 conducted in Africa, which predominantly involved chronic patients. Additionally, a recent meta-analysis73 revealed that the DUP is longest in Africa and South Asia, which, when considered alongside our meta-regression findings showing higher dyskinesia rates with increasing age and DUP, could explain the higher rates observed in these regions. Interestingly, newer studies57 58 conducted in Africa focusing on first-episode patients with shorter DUP have shown lower SMD rates. In contrast, the limited available data for East Asian populations43 49 54 revealed a notably lower prevalence of SMDs, possibly attributable to specific genetic factors akin to those observed in drug-induced movement disorders.18 19 Interestingly, consistent with our observations, data from a meta-analysis by Ali et al
70 on treatment-induced EPSEs showed similar findings. When stratified by continent, patients from Africa had relatively higher drug-induced EPSE rates at 51% (95% CI 47% to 56%), whereas the prevalence was significantly lower in patients from Asia at 13% (95% CI 4.0% to 21%).
Finally, there was considerable variability in the choice of diagnostic tools and case definitions, with some studies using arbitrary thresholds that led to the inclusion of mild or borderline cases as positive cases.39 42 44 49 62 This variability in rating scale choice and case definitions could have contributed to the high heterogeneity of the overall pooled data, although subgroup comparisons stratified by diagnostic tool choice in dyskinesia data did not show significant differences. Furthermore, the reliability of outcome measures was also questioned, as some studies lacked inter-rater comparisons as highlighted in our risk of bias assessment (online supplemental table). In the case of akathisia, which can be a challenging construct in acutely psychotic individuals, some studies used rating scales that only provided a global score,53 57–59 62 while lacking the objective and subjective subdomains found in scales such as BARS.68 Additionally, some rating tools, for example, ESRS,63 are more weighted towards parkinsonism compared with other movement disorders, resulting in variable prevalence rates across studies. On the other hand, due to its predominance among SMDs, parkinsonism may have caused diagnostic overshadowing or ‘masking’ of other conditions,47 such as dystonia, in keeping with its inconsistent reporting across the included studies.
Publication bias
The DOI plots and LFK asymmetry index29 for SP and akathisia data revealed a symmetrical distribution with low LFK indices, −0.03 and −0.32, respectively (online supplemental figures 7 and 8). These findings suggest no evidence of publication bias in the reported data. Conversely, there was a major asymmetry in the DOI plot for SD data (LFK index 3.54), indicating a potential major publication bias (online supplemental file 9).
Further investigation into whether this DOI plot asymmetry stems from a small-study effect involved using the regression test by Peters et al. However, the test did not show a significant relationship between the two variables (t=−0.60, p=0.55), suggesting that the asymmetry observed in the DOI plot may be attributed to factors beyond publication bias, such as high degree of between-study heterogeneity and due to other moderating variables potentially unaccounted for in this meta-analysis.
Finally, it is important to note that traditional publication bias tools are of limited utility and applicability in meta-analyses of proportions.74 Moreover, trim-and-fill methods, commonly used to adjust for publication bias, may even yield invalid results, especially in the presence of high between-study heterogeneity.75 Therefore, we opted not to use this method to prevent any spurious conclusions, given a significant heterogeneity observed in the SD data.
Future perspectives and recommendations
The findings of this systematic review and meta-analysis underscore the importance of recognising pre-existing SMDs as a distinct subtype or symptom cluster within schizophrenia and other psychotic disorders. Spontaneous motor abnormalities have been linked to more severe positive and negative symptoms,38 42 44 45 58–60 76 cognitive dysfunction, poorer treatment response and long-term outcomes.42 49 57 60
It is unclear what effect antipsychotic treatment has on patients with pre-existing SMDs, as it may be linked to a complex mixture of primary and drug-induced features.45 60 Different studies have shown inconsistent outcomes: some indicate worsening of SMDs,41 42 45 49 57–59 62 particularly SP,41 42 45 49 59 especially in the acute treatment phase and with typical antipsychotics, which later tends to subside with long-term treatment. Conversely, some studies report improvement in pre-existing SMDs,49 52–54 59 especially with some atypical antipsychotics with low propensity to cause EPSEs.49 53 54 Finally, some motor abnormalities, such as dyskinesia, appear to re-emerge in the long term, potentially due to the progression of the movement disorder and/or different pathophysiological mechanisms linked to long-term dopamine receptor blockade due to antipsychotic medication.49 58
There is an important need for better recognition of cases with SMDs and the implementation of individualised treatment approaches, especially in the context of the rapidly emerging precision psychiatry. Clinicians are urged to conduct baseline examinations before commencing treatments to inform more appropriate treatment decisions.
Future research should focus on assessing spontaneous movements disorders using valid and reliable methods, while also ensuring robust sampling and inclusion/exclusion criteria to capture a representative population. Additionally, it is crucial to further explore potential moderating variables across various SMDs, such as sex and racial differences, diagnostic variations, age and DUP.