Results
Search results
Figure 5 describes the results of our search. We included 14 studies: 11 studies identified in our updated search and 3 from the previous review20–22 (online supplemental table 1). Four studies were rated as low risk of bias,23–26 one as high risk of bias27; the remaining nine were rated in the intermediate category of ‘some concerns’ (online supplemental table 1).
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram illustrating the process of study selection.
Study description
Seven of the identified studies were conducted in the USA, two in Canada and one each in Australia, France, Israel, The Netherlands and Spain. Participants were mostly recruited via convenience sampling from psychiatric clinics. The mean age of all participants with outcome data available was 65.0 and ranged from 55.228 to 75.9.22 Nine studies specified a minimum age as an inclusion criterion.22–26 29–32 The remaining five included all adults.20 27 28 33 34 Overall, 648 out 1168 (55.5%) of participants were female. No study reported the effect of age or gender on treatment.
Eight studies excluded patients with suspected or confirmed dementia.22–25 28 30–32 Among those that did not, three included cognition as an outcome26 27 33 and two did not.29 34 Six studies excluded anyone expressing acute suicidal intent,23 25–28 30 and three measured suicidal ideation as a secondary outcome.24 28 29 Most studies excluded uncontrolled acute but accepted stable medical conditions. Nine studies excluded anyone with a current or previous psychotic episode or prescribed antipsychotic medication.20 24–26 29–33 Others included people taking antipsychotic medication.23 27 Navarro et al included people with psychotic depression, with 13% of participants prescribed antipsychotic medication at baseline.34 Yesavage et al investigated US veterans with comorbid post-traumatic stress disorder and allowed any concomitant psychiatric medications.28
In addition to requiring evidence of at least one previous treatment failure, three studies confirmed treatment resistance prior to randomisation by conducting a 10–12 week trial of venlafaxine24 34 or an 8-week trial of a selective serotonin receptor inhibitor followed by an 8-week trial of an additional antidepressant.22 Another study prospectively assessed adherence to pre-study antidepressant medication regimen for 4 weeks.26
Overall treatment efficacy
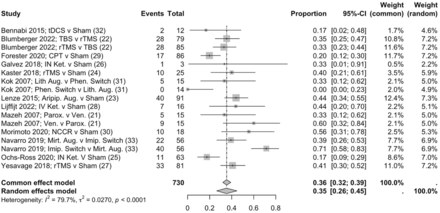
We first investigated the likelihood of achieving remission across all the studies. In our pooled meta-analysis of the proportion of participants in active intervention groups achieving remission, we included 17 active interventions, as four studies compared active treatments.22 30 32 34 We were unable to include one study that did not report remission rates.20 Over study periods of 1–12 weeks, the overall odds of remission was 0.35 (n=17; 95% CI=0.26; 0.45). There was considerable heterogeneity between groups (I2=79.7% (95% CI=66.6%; 88.0%)).
We then compared the odds of achieving remission in active intervention relative to placebo arms. We could not include the four studies that did not have a placebo-control arm,22 23 32 34 nor one placebo-controlled trial as required data were unavailable.20 In our random effects model, the overall OR of 2.42 (n=9; 95% CI=1.49; 3.92) favoured the intervention arms. The funnel plot displayed reasonable symmetry, indicating that there was no significant publication bias and we did not find evidence of heterogeneity (I2 statistic=28.6%).
Only two interventions were studied in more than one RCT: ketamine and interventions involving transcranial magnetic stimulation (TMS). Subgroup analysis findings are discussed below.
Ketamine therapy
Three studies investigated the effects of ketamine therapy on TRD.26 27 29 All defined remission using the Montgomery-Asperg Depression Rating Scale (MADRS) (online supplemental table 1). Gálvez et al27 and Ochs-Ross et al26 administered eight treatments of intranasal (IN) esketamine over 4 weeks, with the control group receiving IN midazolam. Gálvez et al27 used a fixed dose of 100 mg esketamine in a small pilot RCT, for which the primary aim was to evaluate safety and feasibility. Ochs-Ross et al26 used flexible dosing, starting at 28 mg, then increasing to 56 or 84 mg at clinician discretion; for the primary endpoint of MADRS scores, the estimate of the treatment difference (−3.6, 95% CI (−7.20, 0.07)) did not attain significance (figure 3).
Lijffijt et al29 compared one intravenous ketamine infusion to a midazolam control at 7 days post-infusion, with the aim of comparing three doses: 0.1, 0.25 and 0.5 mg/kg. For the purposes of analysis, 0.25 and 0.5 mg/kg groups were combined. Comparable results for the 0.1 mg/kg intervention group were unavailable for inclusion in the meta-analysis.
In our subgroup meta-analysis, the overall OR on the primary outcomes of remission was 2.91 (n=3; 95% CI=1.11; 7.65), favouring the intervention.
GRADE assessment
Ketamine was evaluated in three RCTs. Due to the high risk of bias exhibited in one study27 and inconsistency evidenced by the wide CI in the pooled analysis, we downgraded the quality of evidence for the effects of ketamine on remission by two levels to low, concluding that the quality of evidence is weak for ketamine therapy effects on TRD remission in older people.
TMS therapies
TMS is a form of non-invasive brain stimulation where a brief magnetic field passes through the scalp and induces an electrical current in the cerebral cortex. Four studies tested interventions involving TMS: transcranial direct current stimulation (tDCS), repetitive transcranial magnetic stimulation (rTMS) and theta Burst Stimulation (TBS). Bennabi et al33 compared 10 sessions over 5 days of active or sham tDCS applied to the left dorsal left pre-frontal cortex (DLPFC); intervention relative to control group improvements on Hamilton Depression Rating Scale (HDRS) (33% vs 19%; p=0.17) and MADRS scores (27% vs 15%; p=0.35), and a higher level of response (25% vs 9%; p=0.59) and remission (17% vs 0%; p=0.47) did not attain significance (figure 4).
Three studies investigated rTMS.23 25 28 Two were placebo-controlled trials, both using HDRS as the primary outcome. Kaster et al25 switched from a helmet coil, only stimulating the left DLPFC, to a coil targeting the DLPFC and VLPFC (ventral left pre-frontal cortex) bilaterally, with greater penetration on the left after six patients experienced poor treatment tolerance. Each participant completed 20 sessions over 4 weeks. By contrast, Yesavage et al28 administered five sessions over 12 days, stimulating the DLPFC only. Yesavage et al28 and Kaster et al25 reported suicidality as a secondary outcome, finding a non-significant decrease in the active stimulation groups. Finally, Blumberger et al23 compared two active treatments: rTMS and TBS. Each participant received 20 sessions over 4 weeks. For rTMS, 32.9% achieved remission, and there was an improvement in MADRS and HDRS scores of 32% and 33%, respectively. Results for TBS were similar (35.4% remission; 39% improvement in MADRS; 38% improvement in HDRS).
We meta-analysed findings for these studies comparing stimulation to control. The random-effects model OR was 1.99 (n=3; 95% CI=0.71; 5.61). The I2 statistic indicated a heterogeneity of 44.64%.
GRADE assessment
TMS was evaluated in three RCTs, two rated as having some concerns on risk of bias assessment,28 33 and there was evidence of heterogeneity. Risk of bias, inconsistency and imprecision informed our conclusion that the quality of evidence is very weak for TMS effects on TRD remission in older people.
Other pharmacological interventions
Six studies focused on oral pharmacological interventions for TRD.24 30 34 Lenze et al24 compared 12 weeks of augmentation with aripiprazole to placebo. All patients received venlafaxine extended release, titrated to 300 mg/day. After 12 weeks, those not in remission were randomised to aripiprazole (10–15 mg) or placebo augmentation. On the primary outcome of the MADRS, remission rates were higher in the treatment group (n=181, OR 1.93,1.04–3.58). Lenze et al24 included the Scale of Suicidal Ideation as a secondary outcome; ideation resolved in 22 out of 30 (73.3%) participants presenting with suicidal ideation in the active group at baseline and 11 out of 25 (44%) in the placebo group (p=0.02).
Navarro et al34 compared two active treatments over 10 weeks, with the second group switching their antidepressant rather than adding mirtazapine. Participants who did not remit after 10 weeks of venlafaxine (225–300 mg/day) were randomised to mirtazapine augmentation (30 mg/day) or switch to imipramine (adjusted to plasma levels). 39.28% of those receiving mirtazapine, relative to 71.43% on imipramine, achieved remission (p=0.001).
Forester et al30 evaluated the impact of pharmacogenetic-guided prescribing. Over 24 weeks, clinicians had access to this report for intervention but not control participants. Assessors were only blinded up to week 8, the primary endpoint, where the OR favoured the treatment group (n=184, OR 3.20, 1.26–8.14).
The three RCTs that we included from our previous review were a placebo-controlled trial (evaluating high-dose selegiline)20 and two studies comparing active treatments (phenelzine and lithium, and venlafaxine and paroxetine, respectively).22 32 The placebo-controlled trial employed a cross-over method and found that in the objective measures of depression, symptoms significantly improved following active treatment with selegiline 60 mg/day (n=16; 37.4% decrease in HDRS). For the remaining two studies, lithium was found to show a greater rate of remission compared with phenelzine (n=5; 33%, vs n=0; 0%, respectively) as well as a greater rate of response (n=7; 46.7%, vs n=1; 7.1%).32 Both venlafaxine and paroxetine showed a statistically significant improvement in HAM-D scores, with venlafaxine showing a greater rate of remission (n=9; 60% vs n=5; 33%, respectively).22
GRADE assessment
We downgraded evidence for interventions evaluated in single trials, as we could not judge consistency of findings. Low risk of bias informed our conclusion that the quality of evidence is weak for the effect of aripiprazole augmentation on remission of TRD in older people. We concluded that the quality of evidence for pharmacogenetic testing-guided prescribing, evaluated in a trial rated as having ‘some concerns’ of bias was very weak. The single, placebo-controlled trial of selegiline employed a cross-over method alongside a very small sample size; we concluded there was insufficient evidence to make a GRADE assessment.
Psychological interventions
Neuroplasticity-based computerised cognitive remediation (NCCR)31 involves activities that target the cognitive control deficits thought to influence depression and its poor outcomes in older populations. The authors proposed online activities targeting the processing of sensory stimuli as a depression treatment. In this small, double-blind, randomised trial, the active group was more likely to achieve remission over 4 weeks (n=18; OR 21.25, 2.31–195.64).
GRADE assessment
Risk of bias, inconsistency and imprecision informed our conclusion that the quality of evidence is very weak for the effect of cognitive remediation on TRD remission in older people.