WHAT IS ALREADY KNOWN ON THIS TOPIC
-
The COVID-19 pandemic has resulted in a global increase in anxiety, depression and psychological distress.
-
Mixed findings on sleep patterns have been reported, with some individuals experiencing sleep difficulties and others noting longer sleep durations.
-
While people with mood disorders experienced worsened sleep quality during lockdowns, research on sleep duration changes and their associated factors in people with a history of depression is limited.
WHAT THIS STUDY ADDS
-
This study shows that optimal sleep patterns decreased from 70% pre pandemic to 49% during the pandemic, with shifts towards both short and long sleep.
-
It identifies factors such as mental health conditions, stressful life events, chronotype and genetic susceptibility as influencing transitions to short or long sleep, suggesting possible depressive subtypes.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Introduction
COVID-19 has led to a global increase in mental health concerns and disorders, including rising rates of anxiety, depression, post-traumatic stress disorder (PTSD) and psychological distress,1 2 with an estimated ~10% rise in depressive symptoms.3 Factors including social support, financial difficulties, unemployment, educational attainment and psychological flexibility have been identified as predictors of deteriorating mental health during the pandemic.1 2 Moreover, COVID-19 has significantly altered in sleep patterns in the general population,4 although research into pandemic-driven effects on sleep has yielded mixed findings. On the one hand, in some populations, the pandemic led to an estimated 14.5% increase in sleep difficulties, including disruption to sleep initiation, maintenance and early awakenings,5 which are associated with depression, anxiety and PTSD.6 7 These changes are further substantiated by a recent meta-analysis that revealed a pandemic-driven insomnia prevalence of 23.5%,8 suggesting significantly shorter sleep for many individuals. On the other hand, some studies have reported longer sleep patterns (>7 hours), reduced ‘social jetlag’ (ie, the difference in sleep timing between weekdays and weekends) and increased time in bed dedicated to sleep.9 Interestingly, people with evening chronotypes (ie, so-called night owls) experienced delayed sleep schedules, increased sleep patterns and heightened sleep problems, along with poorer mental health.10 These changes in sleep patterns during the COVID-19 pandemic might be attributable to the implementation of ‘lockdown’ policies or ‘stay-at-home’ orders, allowing for personal preferences to more strongly dictate daily schedules.9 11
The sleep of people experiencing mental health challenges (eg, mood disorders) has also been affected by the pandemic, particularly during ‘lockdown’ periods when, on average, they experienced worsened sleep quality.12 13 This decline in sleep quality was associated with higher rates of depression, anxiety and psychological distress.12 Among those with mood disorders, increased sleep disturbance during the pandemic was associated with elevated social anxiety, alcohol consumption and being in a married or de facto relationship,14 while poor sleep quality exacerbated depression risk among evening chronotypes.15
Despite several studies examining pandemic-induced mental health and sleep disturbances, there is a notable gap in investigating the association between changes in sleep duration—a common clinical complaint or feature among people with mental disorders—and sources of variation in changes in sleep in people with a self-reported history of depression. The COVID-19 pandemic acted as a dual stressor, presenting both non-specific stress and an opportunity for individuals to diverge from conventional social constraints on sleep–wake cycle behaviour. This unique circumstance serves as a behavioural experiment, and in this study, we investigate its effects on a susceptible population with a history of depression. The primary goal of this study is to leverage the pandemic as a natural experiment, examining how individuals with a history of depression adjust their sleep patterns in response to a significant stressor, using the Australian Genetics of Depression Study (AGDS).16 Additionally, we aim to identify sociodemographic, clinical and genetic factors influencing changes in sleep patterns among those with optimal sleep patterns pre pandemic. We hypothesised that different depressive phenotypes would undergo distinct changes in their sleep pattern during COVID-19, as would be predicted by prior research suggesting the presence of distinct pathophysiological subtypes of depression with different mechanistic links to sleep disturbance.17–20 Specifically, we expect individuals with characteristics of ‘anxious depression’ to experience shorter sleep duration, likely due to cognitive and arousal processes (ie, difficulty ‘switching off’ thinking). By contrast, we expect those with characteristics of ‘circadian depression’ will experience longer sleep long duration due to a prepandemic misalignment between their chronotype and social schedule,17 which, if relaxed during the pandemic, allowed a better alignment of sleep–wake timing with biological rhythms.
Methods
Participants and study design
Study participants were members of the AGDS, one of the largest cohorts in the world for exploring genetic and psychosocial factors influencing the aetiology of depression and response to antidepressant medication. A total of 20 689 participants (75% women; mean age 43±15 years, range: 18–90 years) were recruited through letters sent by the Australian Department of Human Services or a media publicity campaign and completed an online survey, and 76% of participants provided saliva samples using a mail-out DNA saliva collection kit. Among the respondents, 98.5% had consulted a professional about their mental health issues, with 93.4% (n=19 803) having been diagnosed with depression. Anxiety disorder was the second most frequently reported diagnosis (55.0%), followed by PTSD (14.0%) and social anxiety disorder (11.4%). Additionally, 95% (n=19 585) of respondents reported using antidepressants, with 93% (n=18 174) taking them for depression and 51% for anxiety. Additional details about recruitment strategy and sampling are provided in a cohort profile.16
The ‘baseline’ (ie, pre pandemic) data were collected between 2016 and 2018, and a subsequent survey was conducted between April 2020 and February 2021 (n=9436). A total of 6453 participants completed both baseline and follow-up surveys, which each included questions about sleep patterns. The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.21
Measures
The analysis drew on a range of self-report variables collected at baseline and follow-up surveys.
Sociodemographic factors:
-
Baseline: age, sex.
-
Follow-up: marital status, highest education levels, household income and work status.
Mental health-related measures (self-report; collected at baseline only):
-
Somatic and Psychological Health Report (SPHERE-12)22: 12-item scale assessing somatic and psychological symptoms of depression (higher scores indicate higher distress).
-
Kessler Psychological Distress Scale (K10)23: 10-item scale measuring psychological distress (higher scores indicate higher distress).
-
Suicidal Ideation Attributes Scale (SIDAS)24: 5-item questionnaire evaluating suicidal ideation attributes (higher scores indicate more severe suicidal thoughts).
-
Altman Self-Rating Mania Scale
25: 5-item scale assessing manic symptoms (higher scores indicate a higher probability of a manic or hypomanic condition). -
Community Assessment of Psychic Experiences (CAPE) (modified version)26: 6-item scale assessing psychotic-like symptoms (higher scores indicate higher severity of psychotic-like experiences).
-
Life Events Checklist for DSM-5
27: 16-item measure estimating stressful life events (SLEs) (higher scores indicate a greater number of SLEs).
Sleep-related measures (self-report; collected at baseline):
-
Morningness–Eveningness Questionnaire, reduced version (rMEQ)28: 5-item questionnaire (higher scores indicate greater tendency towards morningness).
-
Insomnia Severity Index (ISI)29: 7-item questionnaire measuring insomnia (higher scores indicating greater severity of insomnia).
-
Self-reported actual sleep duration during the past month (hours): we classified categories as: ‘short sleep’ (<6 hours), ‘optimal sleep’ (6–8 hours) and ‘long sleep’ (>8 hours).
Collected at follow-up:
Sleep duration in the last 2 weeks: response options included <6 hours, 6–8 hours, 8–10 hours or >10 hours. We classified categories as: ‘short sleep’ (<6 hours), ‘optimal sleep’ (6–8 hours) and ‘long sleep’ (>8 hours).
Polygenic scores (PGS)
DNA samples were collected using saliva kits, genotyped using the Illumina Global Screening Array V.2.0, and processed in accordance with quality control measures. Samples were merged with the 1000 Genomes project samples30 and principal components were calculated using a set of unlinked single-nucleotide polymorphisms (SNPs). Preimputation quality control was done using PLINK V.1.9.31 32 Quality control involved removing SNPs with a minor allele frequency<0.005, an SNP call rate<97.5% and identification of participants with genetic similarity33 to a European reference group (>4 SD from ancestry principal components (PCs) PC1/PC2 centroid) and Hardy-Weinberg equilibrium (p<1×10−6), before imputation using the Haplotype Reference Consortium 1.1 reference panel.34 Genome-wide association studies informed SNPs associated with mental disorders and related traits: major depressive disorder (MDD),35 bipolar disorder,36 schizophrenia,37 neuroticism,38 attention-deficit/hyperactivity disorder,39 anxiety,40 insomnia41 and circadian preference (chronotype).42 Where applicable, leave-one-out summary statistics were used for Genome-wide association studies that included participants from the AGDS in order to avoid over-estimation of association results. sBayesR, a Bayesian PGS method, was used to generate allele weights for each PGS.43 The posterior SNP effects for each disorder and trait were used to generate PGS for each participant using the PLINK score function.31 The scale() function was used to standardise the PGS for analysis. Effect sizes are interpretable as reflecting a 1 SD increase or decrease in each PGS.
Statistical analysis
Statistical analyses were conducted in RStudio using R (V.4.2.3).44 Based on responses to the sleep duration questions at baseline and follow-up, we created three groups at baseline and follow-up (‘short sleep’, ‘long sleep’ and ‘optimal sleep’), and nine possible trajectories between these groups. We focused our main analyses on examining the group with ‘optimal sleep’ at baseline, from which three trajectories were possible: ‘maintain optimal sleep’, ‘optimal-to-short sleep’ and ‘optimal-to-long sleep’. Analyses of the other groups are available in the online supplemental materials. Differences among sleep pattern groups in sociodemographic, clinical and sleep variables were assessed via analysis of variance (ANOVA) or χ2 tests where relevant. ANOVA with Dunnett’s post hoc tests was used to test significant differences against the ‘maintain optimal sleep’ group, where applicable. All statistical tests were two-tailed and used a significance level of α=0.05. Multinomial logistic regressions were conducted using the R ‘nnet’ package with the ‘multinom’ function to examine sociodemographic, mental health, sleep and genetic predictors (predictor variables) of membership in the sleep transition groups (outcome variable), where the reference category was ‘maintain optimal’ for optimal sleepers at baseline and ‘shift to optimal’ for short or long sleepers at baseline. We report unadjusted and adjusted models, controlling for relevant variables (sociodemographic, mental health, sleep and PGS). To control for multiple comparisons in the multivariate analyses, we report uncorrected p values and false discovery rate (FDR)-corrected p values (pFDR). Missing data were excluded from the analysis.
Results
Changes in sleep patterns during the COVID-19 pandemic
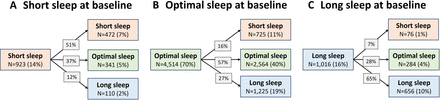
Figure 1 shows the significant shifts in sleep trajectories before (2016–2018) and during the COVID-19 pandemic (2020–2021) (χ2=1244.9, p<0.001). Notably, before the pandemic (at baseline), most of the sample (70%, n=4515) had ‘optimal sleep’ pattern; however, this fell to about half (49%, n=3189) during the pandemic. The proportion of ‘long sleepers’ nearly doubled from 16% (n=1016) to 31% (n=1991). Similarly, ‘short sleep’ increased from 14% (n=923) to 20% (n=1273). While approximately half of those with ‘short sleep’ or ‘long sleep’ maintained their sleep patterns after the pandemic, a subset (<10%) improved their sleep, shifting an optimal sleep pattern.


Transitions in self-reported sleep pattern across the COVID-19 pandemic in the Australian Genetics of Depression Study (n=6453 with longitudinal data).
While we could elaborate on a variety of sleep trajectories (figure 1), this report specifically focuses on understanding why certain people with a previous ‘optimal sleep’ pattern displayed resilience, maintaining their sleep patterns, while others transitioned to less adaptive sleep (short/long), even when afforded greater flexibility in choosing their sleep–wake schedules. Descriptive characteristics and statistical models pertaining to those who reported ‘short sleep’ or ‘long sleep’ before the pandemic are available in the online supplemental materials.
Characteristics of prepandemic ‘optimal sleepers’
Among 4514 people who reported optimal sleep pattern before the pandemic, over half (57%, n=2564) maintained their optimal sleep pattern (henceforth referred to as ‘maintain optimal sleep’), while 16% (n=725) transitioned to short sleep (henceforth, ‘optimal-to-short sleep’) and 27% (n=1225) shifted to long sleep (henceforth, ‘optimal-to-long sleep’). Table 1 summarises the prepandemic characteristics of these three groups including sociodemographic, mental health, sleep-related characteristics and genetic risk scores.
Prepandemic characteristics of the sleep trajectory groups among participants who reported having optimal sleep pattern before COVID-19 (n=4514)
The ‘optimal-to-short sleep’ trajectory had a slightly higher proportion of women (78% vs 74%–75%; p=0.05, table 1). During COVID-19, based on the postpandemic survey, this group had fewer individuals with partners (p=0.002), a lower rate of engagement in paid work (p<0.001) and the highest proportion in the lowest income band (p=0.001), compared with the other trajectory groups (online supplemental table S1). Notably, compared with the ‘maintain optimal sleep’ group, the ‘optimal-to-short sleep’ group reported poorer mental health before the pandemic, as evidenced by higher psychotic-like symptoms (CAPE; p=0.004); higher somatic and psychological symptoms (SPHERE; p<0.001); increased psychological distress (K10, p<0.001); and higher suicidality risk (SIDAS; p<0.001). They also reported more exposure to SLEs (p<0.001) and higher baseline insomnia symptoms (ISI; p<0.001). Although genetic risk scores were similar across the three trajectory groups, those maintaining optimal sleep had the lowest PGS, and the ‘optimal-to-short sleep’ group had a higher depression polygenic score than those maintaining optimal sleep (p=0.002).
As shown in table 1, individuals in the ‘optimal-to-long sleep’ trajectory were slightly younger than the other groups, particularly compared with the ‘maintain optimal sleep’ trajectory (p<0.01). Before the pandemic, this group had significantly poorer somatic and psychological symptoms (SPHERE; p=0.001); increased psychological distress (K10, p=0.013); and a higher propensity for being evening types (rMEQ, p<0.001). Additionally, they had a significantly higher depression polygenic score than the ‘maintain optimal sleep’ trajectory (p=0.04).
Predictors for sleep pattern trajectories (multivariate models)
Among prepandemic optimal sleepers, the ‘optimal-to-short sleep’ trajectory was significantly associated with a higher likelihood of reported SLEs (adjusted OR (aOR)=1.09 (95% CI:1.05 to 1.14), pFDR<0.001), higher SPHERE scores (aOR=1.03 (95% CI: 1.01 to 1.05), pFDR=0.004) and higher ISI scores (aOR=1.06 (95% CI: 1.03 to 1.08), pFDR<0.001). Moreover, a higher depression polygenic score was associated with the ‘optimal-to-short sleep’ trajectory, although this association did not retain statistical significance after applying the FDR correction (aOR=1.11 (95% CI: 1.00 to 1.24), p=0.043, pFDR=0.183). In the unadjusted model, lower income and a higher schizophrenia PGS were also associated with ‘optimal-to-short sleep’ trajectory, but these associations were not significant in the adjusted model.
Conversely, no FDR-corrected significant predictors emerged for the ‘optimal-to-long sleep’ trajectory. Before FDR correction, a greater propensity for eveningness (rMEQ) (aOR=0.98 (95% CI: 0.96 to 1.00), p=0.031, pFDR=0.183) and a higher MDD PGS (aOR=1.09 (95% CI: 1.00 to 1.19), p=0.046, pFDR=0.183) were associated with the ‘optimal-to-long sleep’. PGS for other psychiatric disorders or insomnia were not significant predictors (table 2).
Multinomial logistic regression comparing predictors of sleep trajectories to the ‘maintain optimal sleep’ reference group in people with initially optimal sleep
Discussion
In this study, we explored changes in sleep pattern during the COVID-19 pandemic in a genetically informative cohort of 6453 adults with a self-reported lifetime history of depression. This unique natural experiment unveiled significant shifts in sleep patterns. Despite increased flexibility to adjust their sleep schedules due to ‘lockdown’ regulations, the proportion of individuals with optimal sleep decreased, leading to transitions towards both short and long sleep patterns. These deviations were influenced by a complex interplay of clinical and genetic factors, including exposure to SLEs, psychological or somatic distress, chronotype, insomnia severity and genetic susceptibility to MDD.
The regression model partially supports our hypothesis that distinct depressive phenotypes undergo specific sleep pattern changes during COVID-19. A shift towards short sleep was related to prepandemic vulnerability to sleep problems (ie, higher insomnia), poorer mental health (ie, higher somatic/psychological distress) and higher (but marginal) depression genetic risk. Potentially, these factors may be linked to social support and coping, evident by a higher likelihood of reported exposure to SLEs. By contrast, while a shift towards longer sleep had poorer prepandemic mental health, a greater propensity for eveningness, and a higher depression genetic risk, these factors were not statistically significant after FDR correction. We speculate that before the pandemic, these individuals had social or work schedules that were not aligned with their biological circadian preference, and when able to relax these schedules (ie, during lockdowns), these individuals shifted towards a sleep–wake schedule more ‘natural’ to them.
While a U-shaped relationship between sleep duration and mental health has been well established in previous research (ie, both short and long sleep being associated with the risk of depression or psychological stress),45 46 our findings indicate that this relationship was primarily associated with the transition from optimal to short sleep as shown in the significant links with more exposure to SLEs, heightened somatic or psychological distress and elevated insomnia symptoms. Although the ‘Optimal-to-Long sleep’ trajectory also exhibited poorer mental health compared with those maintaining optimal sleep, the extent was not as pronounced as observed in individuals shifting to short sleep. Instead, we observed that a greater eveningness may be linked to membership in the ‘optimal-to-long sleep’ group, consistent with previous research indicating that individuals with evening chronotypes experienced extended sleep durations during the COVID-19 pandemic.10 This phenomenon may be attributable to evening types adjusting their sleep–wake schedules based on their inherent circadian preferences, thus increasing their sleep pattern in a flexible environment facilitated by the COVID-19 pandemic (eg, working from home, reduced schedule constraints). This is consistent with our proposed ‘circadian depression’ subtype, characterised by a prolonged sleep period associated with an evening chronotype.17 20 However, it is important to note that this relationship was observed only in self-reported chronotype and not in the genetic chronotype measures.
The inclusion of depression PGS as a predictor in our regression models offers intriguing insights into the genetic underpinnings of sleep trajectories. The results suggest a modest yet potentially meaningful association between depression genetic risk and sleep trajectories. While the OR and CI indicate a reliable effect size, it is noteworthy that the FDR did not reach significance after correcting for multiple testing, emphasising the need for cautious interpretation considering factors such as sample size and effect size. This observed association implies a genetic link between MDD susceptibility and sleep patterns, warranting further exploration in larger cohorts and replication studies to unravel the nuanced interplay between genetic factors and sleep trajectories (particularly as more variants are identified and the depression genetic risk scores becomes more powerful). Consistent with other genetic studies demonstrating increased genetic influence on depressive symptoms in individuals with both short and long sleep patterns,47–49 our findings shed light on the higher MDD genetic risk observed in groups transitioning from optimal sleep to either short or long sleep following the COVID-19 pandemic. Furthermore, a prior study in the same AGDS cohort established an association between higher genetic liability to MDD and an increased likelihood of comorbid anxiety disorders.50 The ‘optimal-to-short sleep’ group, in contrast to the ‘optimal-to-long sleep’ trajectory, exhibited significantly poorer mental health with heightened anxiety levels. Taken in conjunction with our previous research, it is reasonable to posit that the ‘optimal-to-short sleep’ group shares characteristics with an ‘anxious depression’ subtype, typified by elevated depression genetic risk, increased insomnia and higher levels of anxiety.17 Speculatively, these individuals may be more vulnerable to reductions in sleep pattern in response to major stressors.
Some limitations should be acknowledged. The study focused on changes in sleep duration and did not consider shifts in sleep timing, which may have been significantly affected by the pandemic (eg, delaying sleep onset/offset times). Additionally, the study’s reliance on self-report rather than objective sleep–wake measurements (eg, actigraphy) may introduce recall bias. Notably, different mental health assessments were employed in the two surveys, rendering a direct comparison of their mental health changes following the pandemic unfeasible. Last, the transitions in sleep patterns during the pandemic may, in part, be attributed to changes in structural constraints, such as family-related stressors such as caregiving and home-schooling responsibilities, or the limited social structures for individuals living alone during lockdowns. However, given the survey spanned different periods of lockdown restrictions across Australian states (potentially influencing sleep patterns and daily social rhythms) these factors were not examined. Finally, while those who shifted to shorter or longer sleep showed different average sleep and mental health outcomes, these sleep changes were measured at only two time points and mental health measures assessed at a single time point were included as predictors. This limits our ability to confirm whether these shifts in sleep align with specific illness subtypes, such as anxious depression or ‘circadian depression’, which would require more detailed clinical characterisation of the current presentation and the history of their depressive and other disorders.
In summary, adults with a history of depression with more severe somatic and psychological symptoms of depression, insomnia, eveningness and genetic liability to depression tended to have suboptimal sleep pattern during the COVID-19 pandemic. Particularly, people with features suggestive of an ‘anxious depression’ subtype were more likely to shift towards short sleep, while those with features suggestive of a ‘circadian depression’ subtype were more likely to shift towards longer sleep pattern. Our study unveiled partial insights into the specific connections between alterations in sleep patterns and diverse depressive phenotypes among individuals with depressive disorders. Understanding these dynamics may inform the selection of interventions for those with depression facing major challenges.
Data availability statement
Data are available upon request. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants. Ethical approval was obtained from the QIMR Berghofer Medical Research Institute Human Research Ethics Committee in Brisbane, Australia. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We are indebted to all of the participants for giving their time to contribute to this study. We thank all the people who helped in the conception, implementation, beta testing, media campaign and data cleaning, especially Dr Shin Ho Park. We would like to thank the research participants and employees of 23andMe for making this work possible. The study protocol used by 23andMe was approved by an external AAHRPP-accredited institutional review board.