WHAT IS ALREADY KNOWN ON THIS TOPIC
-
Cognitive impairments in patients with Parkinson’s disease (PD) psychosis are associated with poor quality of life and increased risk of dementia.
-
Research studies have focused on cross-sectional designs and only a handful of studies examined differences in cognitive performance longitudinally in PD psychosis; therefore, the pattern of cognitive decline in PD psychosis remains unclear.
WHAT THIS STUDY ADDS
-
By employing data from the Parkinson’s Progression Marker Initiative study, we examined the trajectory of changes in performance across multiple cognitive assessments in patients with PD with and without psychosis over the course of 5 years.
-
Patients with PD psychosis reported a worse decline in cognitive performance compared with patients with PD without psychosis, specifically in semantic fluency, processing speed, general cognitive functions as well as visuospatial abilities, immediate and delayed recall.
-
These changes were independent of sociodemographics, depression, sleep-related problems and motor symptom severity.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
-
These findings emphasise the importance of investigating cognitive domains, especially semantic aspects of language in PD psychosis.
-
This research highlights the need for early detection and intervention strategies targeting cognitive impairments in PD psychosis to improve patient outcomes and inform clinical practice.
Background
Psychotic symptoms such as hallucinations and delusions are some of the most common and debilitating non-motor symptoms in patients with Parkinson’s disease (PD).1 2 People with PD psychosis (PDP) have poor quality of life and are at increased risk of hospitalisation and dementia.3 4 PDP is associated with cognitive deficits such as impairments in attention, global cognition, executive functions, and processing speed, which may further lead to deterioration in quality of life and increased risk of dementia.5 6
It is clinically recognised that people with PD psychosis report worse cognitive performance compared with patients with PD who do not have such symptoms,7–9 consistent with two recent meta-analyses.10 11 Pooling data from cross-sectional comparisons, they found that functioning across all major cognitive domains, that is, attention, global cognition, memory, perception and executive functions, is impaired in PDP compared with patients with PD without psychosis, with executive function and attention being the most affected domains.10 11 They also reported that age at testing,10 11 depression and PD duration11 had a moderating effect on some of these deficits. Specifically, episodic memory was the most likely impaired domain in PDP as shown by a subgroup analysis conducted in the same samples of patients with a PD duration of 6–9 years. Encoding and retrieval, domains of episodic memory,12 phonemic and semantic fluency, perception (namely dorsal and ventral stream associated visuospatial abilities and low-level visual apperception), construction (namely copying) were also impaired in PDP compared with patients with PD without psychosis, suggesting a potential role of these domains in the symptomatology of psychosis in PDP. However, inference on the progression of cognitive functioning over time is challenging on the basis of evidence from cross-sectional studies. Among the handful of longitudinal studies in this field, the Parkinson and non-motor symptoms (PRIAMO) study13 showed that patients with PD who developed psychosis symptoms (n=37) had worse performance in global cognition (as indexed using the Mini Mental State Examination (MMSE)) at 12 months and 24 months follow-up. Similarly, in their clinic-based retrospective sample, Muller et al
14 found that patients with PD who had visual hallucinations (n=18) at follow-up (mean follow-up duration: 2.8 years) had worse performance at baseline and follow-up compared with patients with PD without psychosis (n=15; mean follow-up duration: 4.7 years) on the measures of processing speed and more broadly on executive functions. Goetz et al
15 also examined differences between patients with PD with and without hallucinations over a period of 6 years. 37 out of 60 patients with PD developed hallucinations across different sensory modalities (eg, visual vs non-visual), however these two groups did not differ on cognition as measured with the MMSE after 6 years from baseline. Using data from the Parkinson’s Progression Markers Initiative (PPMI) study16 (n=131 patients with PD), a 5-year longitudinal study in patients with de novo PD, another study found that a higher proportion of those who developed psychotic symptoms reported subjective cognitive decline compared with those who did not, without any significant group difference in cognitive task performance at follow-up.17 Using the same PPMI study, Ffytche et al
18 showed that 115 patients with PD who developed minor illusions (onset at 19.5 months follow-up) differed on neuropsychiatric symptoms, and olfaction at baseline compared with patients with PD who never developed such symptoms. Although there was no difference in the slope of cognitive decline prior to developing psychosis symptoms between groups, those who developed more severe symptoms of psychosis (n=21, 4.9%) also showed worse performance on the Benton Judgement of Line Orientation (BJLOT) compared with patients with PD, at baseline.
Objectives
While it is generally accepted that there is a progressive decline in cognitive function in people with PD and that this may be greater in patients with PD with psychosis,19–21 to the best of our knowledge, the longitudinal course of cognition has not been systematically examined before. Therefore, to address this gap in evidence here we compare the longitudinal course of cognitive task performance across a range of measures over the 5 years of follow-up from cohort inception in people recruited into the PPMI study. Based on previous literature, we predicted that cognitive task performance will show a greater decline in patients with PD compared with healthy individuals. Our main hypothesis of interest was that this decline will be even greater in patients with PD who later on develop psychosis compared with those who do not develop psychosis over the follow-up period, even after controlling for potential confounders.
Methods
Study design and participants
The PPMI study enrolled newly diagnosed unmedicated patients with PD and age-matched and gender-matched healthy controls. Details of eligibility criteria, objectives and methodology have been published elsewhere16 and can be also found on www.ppmi-info.org/study-design (ClinicalTrials.gov NCT01141023). In brief, patients with PD included were drug-naïve, within 2 years of PD diagnosis, with a Hoehn and Yahr stage <3, 30 years of age or older, had either at least two of resting tremors, bradykinesia or rigidity (must have either resting tremor or bradykinesia) or a single asymmetric resting tremor or asymmetric bradykinesia. Age-matched and sex-matched healthy controls (HCs) were included if they were 30 years of age or older, with no evidence of neurological disorder or a first-degree relative with PD. These data were accessed and downloaded on 1 February 2023 (online supplemental material 1, eFigure 1).16 22 The data used in this analysis are openly available from the PPMI study. Patients with PD were classified into PD with psychosis and without psychosis based on previous work18 using the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part I hallucinations/psychosis item, which measures the presence of visual hallucinations and paranoid thoughts. In brief, if patients with PD reported a score ≥1 at any of the study visits, they were considered as PDP (ie, with psychosis). We have excluded patients with PD who reported psychosis symptoms at baseline (n=33) because the purpose of this study was to examine the decline in patients with PD with and without psychosis with both groups starting off on similar clinical and cognitive characteristics. Three groups were therefore created: HC (no PD symptoms); PDnP: patients with PD with a score of 0, that is, without reports of psychotic symptoms throughout the study and PDP: patients with PD with a score ≥1, that is, with presence of psychosis symptoms which included hallucinations and delusions, at any time point throughout the study. The PPMI study included age-matched and sex-matched HC who were included in the present analysis. Please refer to online supplemental material 1 for more information on sample features and outcome measures. In brief, cognitive outcome measures of interest for the present analyses were: the Montreal Cognitive Assessment (MoCA) (to assess global cognition),23 the Hopkins Verbal Learning Test-Revised (HVLT-R)24 (to assess episodic memory), Symbol Digit Modality (SDM)25 26 (assessing processing speed), Letter Number Sequence (LNS)27 (assessing working memory (ie, manipulation)), Semantic Fluency tests28 and BJLOT29 (assessing visuospatial ability). We also included the following outcome measures as covariates in the analyses: MDS-UPDRS part III30 to measure PD motor symptoms severity (including measures of tremors and rigidity), the Geriatric Depression Scale 15 items31 to measure depressive symptoms; the Epworth Sleepiness Scale and a rapid eye movement sleep behaviour disorder (RBD) questionnaire to measure sleepiness and RBD, respectively.32 33
Statistical analysis
Baseline characteristics were analysed using analysis of variance (ANOVA) or its non-parametric equivalent (eg, Kruskal-Wallis χ2) as appropriate. Post hoc tests were conducted with pairwise t-test with Bonferroni correction for multiple comparisons (or its non-parametric equivalent, ie, Wilcoxon signed-rank test). We examined all study visits from baseline to final follow-up at year 5. We examined the longitudinal trajectory of performance in the HVLT-R, SDM, semantic fluency test, LNS, BJLOT and MoCA and how that differed between HC, PDP and PDnP employing linear mixed-effect analyses with restricted maximum likelihood using the lmerTest package34 in R (V.4.0.3).35 In all analyses, we entered group as a between-subject factor (ie, HC, PDnP and PDP) and year (ie, treated as continuous variable, year 0–5) as a within-subject factor, and patient number as a random effect variable. Significance (a two-sided alpha level of 0.05) was estimated using Satterthwaite’s method. For all cognitive tasks, we first tested a simple (unadjusted) model and then included confounders of interest (ie, age, sex, ethnicity, years of education, depression, sleep-related issues and motor symptom severity) in a separate linear model. We included the scores at each time point (ie, study year) as opposed to only baseline scores for all relevant clinical covariates to ensure that reported results control for longitudinal change in these covariates that might otherwise account for cognitive performance differences. PD medications expressed in Levodopa equivalent daily dose (LEDD) (mg/day) were not included in the model, as we did not find any difference in the amount of LEDD or in the trajectory of dopamine-replacement medications between patients with PDnP and PDP (online supplemental material 1, eTable 1 and eFigure 2). Treatment for psychosis in PD can include administration of antipsychotics such as clozapine, quetiapine and olanzapine,36 37 and antipsychotic treatment has shown to affect cognitive functions.38 39 38 patients were reported to take antipsychotics (online supplemental material 1, eTable 2). We carried out analyses both including and excluding those 38 participants. As the results did not materially differ, we present here the results of analyses including those on antipsychotics; results of analyses excluding them are available on request. All included covariates of interest were mean-centred for analysis.
Findings
Baseline characteristics
Baseline differences between patients with PD and HC are reported in table 1. In brief, patients with PDP reported more severe motor symptoms and more severe PD-related symptoms, more sleepiness, more RBD, more anxiety and more autonomic symptoms than PDnP at baseline. Both PD groups did not differ on any cognitive measures at baseline. They reported more depressive symptoms compared with HC and reported more RBD, more anxiety and more autonomic symptoms than HC. Patients with PD performed significantly worse than HC in the majority of cognitive batteries (all p<0.05) (table 1) (refer to online supplemental material 2 for number of patients with PD who developed psychosis over 5 years, and refer to online supplemental material 2, eTable 4 and eTable 5 for more information on ‘baseline differences’).
Sample characteristics at baseline of the three groups, that is, patients with PD without psychosis (PDnP), patients with PD with psychosis (PDP) and healthy controls (HC). PD psychosis group does not include patients with PD who reported psychosis symptoms at baseline. Mean and SDs are reported, unless otherwise specified
Trajectories of cognitive performance across groups
HC versus PDP and PDnP
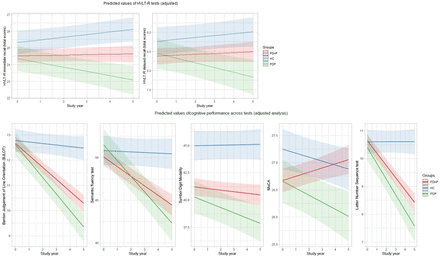
Tables 2 and 3 report the results from the unadjusted and adjusted analyses, respectively. Figure 1 reports the predicted values of task performances for the major cognitive domains. As expected, there was a significant main effect of group across all time points (ie, year 0 to year 5) showing a worse performance of PD groups over HC across HVLT-R tests (ie, immediate recall, and delayed recall, both p<0.001, and recognition, p=0.006 (PDnP), p=0.007 (PDP)). Results showed a worse performance of patients with PD compared with HC on semantic fluency (PDnP vs HC, p=0.006), LNS (PDP vs HC, p=0.015), SDM and MoCA (all p<0.001). Patients with PD also reported a significantly worse trajectory of cognitive decline compared with HC across all tests, except for recognition (PDnP, p=0.208; PDP, p=0.221), and delayed recall (PDP, p=0.08) (table 2). After controlling for covariates of interest, the longitudinal trajectories of task performance across the majority of cognitive measures remained significantly worse in patients with PD compared with HC (online supplemental material 3, eTable 6).
Results from unadjusted group comparison of cognitive performance trajectory between HC, PDnP and PDP
Results from adjusted comparison of cognitive performance trajectories: for ease of presentation, only PDP versus PDnP comparisons reported here (for comparison between HC and PD group, please refer to the online supplemental material 3, eTable 6A, 6B). Analyses were adjusted for depression, sleepiness, REM sleep behaviour disorder and motor symptoms. In bold, significant results pertaining to the main effect and interaction (group × time)


Predictive values from the adjusted analyses. HC,healthy control; HVLT-R, Hopkins Verbal Learning Test-Revised; MoCA, Montreal Cognitive Assessment; PD, Parkinson’s disease; PDP, patients with PD with psychosis; PDnP, patients with PD without psychosis.
PDP and PDnP
In terms of our main hypothesis of interest, unadjusted analyses showed a significant difference in the trajectory of cognitive performance between PDP and PDnP across most measures (ie, interaction of group×time) over the 5-year follow-up period. Patients with PDP showed a significantly worse trajectory over time compared with patients with PDnP across all cognitive tasks, except recognition (p=0.610), and discrimination (p=0.081). After adjusting for age, sex, ethnicity, years of education, depression, sleepiness, RBD and severity of motor symptoms, the longitudinal trajectories remained significantly different between PDP and PDnP for most of the cognitive measures, namely HVLT-R immediate (b=−0.288, 95% CI −0.452 to –0.100, p=0.003) and delayed recall (b=−0.146, 95% CI −0.243 to –0.049, p=0.003), BJLOT (b=−0.178, 95% CI −0.318 to –0.039, p=0.012), semantic fluency (b=−0.704, 95% CI −1.142 to –0.267, p=0.002), SDM (b=−0.337, 95% CI −0.639 to –0.035, p=0.029) and MoCA (b=−0.206, 95% CI −0.300 to –0.111, p<0.001). There was also a trend towards a difference in longitudinal trajectory of LNS between patients with PDP and PDnP (p=0.058).
Discussion
In this study, we investigated the trajectory of cognitive function in patients with PD with and without psychosis and HC. As expected, and in line with previous reports,13 14 21 both groups of patients with PD showed a progressive worsening of performance across a range of cognitive domains, specifically, semantic aspects of language, processing speed, memory, visuospatial and general cognitive abilities, during the early years of their clinical course compared with age-matched and sex-matched healthy individuals. These differences were evident even after controlling for sociodemographic factors (ie, age, sex, ethnicity, years of education) as well as the longitudinal course of clinical factors that may potentially account for these different trajectories. Consistent with our main overarching hypothesis of interest, we found that participants with PD psychosis showed a progressively greater deterioration over time in memory (ie, HVLT-R immediate and delayed recall), processing speed (SDM), language (semantic fluency), visuospatial abilities (BJLOT) and general global cognition (MoCA) compared with participants with PD without psychosis. These effects were evident even after controlling for the potential confounding effects of age, sex, ethnicity and years of education as well as the longitudinal course of depression, sleepiness, RBD and severity of motor symptoms over the same period in those participants. Such a differential trajectory of worsening was not evident for cognitive domains such as recognition and discrimination components of memory, and working memory, namely manipulation (as measured with the LNS). Our findings are broadly in line with previous meta-analytic evidence10 11 from cross-sectional studies indicating impairments in global cognition, visuospatial, language, processing speed as well as in subdomains such as manipulation, immediate recall, category-based fluency in patients with PD with psychosis compared with those without. However, as these analyses were based on cross-sectional studies, the longitudinal course of these impairments remained unclear. To the best of our knowledge, only a small number of studies to date have investigated the longitudinal course of cognition in PD psychosis. While Morgante et al
13 reported lower global cognition as indexed by MMSE scores at 2-year follow-up in patients with PD who developed psychosis, Goetz et al
15 did not find a similar effect. However, Muller et al
14 found poorer performance in processing speed and executive function at follow-up in patients with PD who developed visual hallucinations (n=18) compared with those who did not (n=15). In a large prospective cohort of participants with PD (n=676), here we extend these findings to show that compared with healthy volunteers (n=187), there is a progressive impairment in a number of domains of cognition in people with PD without psychosis at baseline and that this is even greater in people who go on to develop psychosis over the first 5 years following presentation to clinical services, despite both groups of participants with PD being comparable in terms of cognitive task performance at the initiation of cohort. Furthermore, this worsening trajectory of cognition could not be explained by group differences in sociodemographic factors or longitudinal course of potential clinical confounders. The association between psychosis in people with PD and development of dementia is well-recognised.40 Although we excluded from analysis participants who developed dementia during the 5-year follow-up period of the study, we cannot be certain that some of the group-level cognitive impairments observed in participants with PDP were not early indicators of the impending transition to dementia. On average, task performance in the PDP group was at least 1 SD below that in the HC group in more than one cognitive domain, particularly during the latter period of follow-up of the PPMI cohort. Hence, it is possible that many of them may have met the MDS PD-mild cognitive impairment diagnostic criteria.40 41 Results presented here, therefore underscore the need to investigate the relationship between progressive decline in these cognitive domains in people with PDP and eventual clinical diagnosis of dementia in future studies.
Interestingly, our findings highlight the greater progressive worsening in semantic fluency, a measure of semantic aspects of language, in patients with PD who develop psychosis compared with those who do not, which is consistent with previous evidence of language deficits in hallucinating patients with PD.5 6 Semantic decline as revealed by similar tests to those used in the PPMI cohort is a core feature of fronto-temporal dementia and other neurodegenerative conditions.42–44 Consistent with our results, impaired baseline semantic fluency45 46 in conjunction with suboptimal performance in symbol digit modality, and recall (as measured with the HVLT-R) tasks47 have been shown to predict cognitive decline in patients with PD. Semantic deficits in these conditions are associated with Alzheimer’s disease (AD) neuropathology, primarily in the anterior temporal lobe. Although the underlying neuropathology differs, the same anterior temporal cortex region, particularly the amygdala, is associated with visual hallucination in the context of PD or dementia with Lewy Bodies.48 49 Our findings show that patients with PD who develop psychosis report a greater decline in semantic fluency compared with patients with PD without psychosis. While speculative, the initial changes observed in PD psychosis may involve Lewy Body neuropathology within the anterior temporal lobe,48 suggesting that a combination of Lewy Body and AD neuropathology may be presumably responsible for both semantic fluency decline and onset of PD psychosis. However, the role of decline in semantic fluency in PD psychosis requires further investigations. We also observed greater progressive worsening in PDP compared with PDnP in processing speed, which is consistent with the suggestion that visual hallucinations in PD may be a result of dysfunctional attentional processing due to impaired attentional networks such as the ventral and dorsal attentional networks.50–52 As we have observed declining performance across visuospatial abilities, overall general cognitive abilities (as measured with the MoCA) and memory (namely immediate and delayed recall), attributing the emergence of psychosis in PD solely to dysfunctions and altered connections between dorsal and ventral attention networks might oversimplify the complex brain mechanisms involved. Notwithstanding the relevance of the current model of visual hallucinations proposed by Collerton et al,52 the present work may seem to suggest that visual hallucination, or more generally psychosis, may not solely be due to visual processing deficits and impairments in attention. Semantic fluency or semantic loss should be considered as part of this model, as a symptom in PD psychosis and included in the model for a more comprehensive understanding. In line with this, it is important to remember that we do not know yet if semantic deficits preceded visual deficits or vice versa, therefore this needs to be tested. Similarly, Collerton et al
52 and Shine et al
51 53 models emphasise the involvement of dorsal and ventral networks, as well as the Default Mode Network in PD psychosis. Our results suggest including the anterior temporal lobe and semantic aspects of language in the model. Collectively, this warrants more examination to obtain insights in temporal regions potentially affected in PD psychosis.
Given the prevalence of psychosis in PD is 20%–30%,54 our findings further highlight the urgency of prioritising treatment for cognitive impairments. Hallucinations and cognitive deficits may lead to poor quality of life,55 namely poor verbal fluency is associated with deteriorating health and increased care burden in PD.56 Preventative measures such as cholinesterase inhibitors,57 58 especially rivastigmine, are commonly used in PD for psychosis and cognitive impairment59 and have demonstrated benefits in the domains of praxis, memory and executive functioning in neurodegenerative disorders.60 61 Notably, our findings align with the report by Aarsland et al,40 indicating that the observed longitudinal changes in performance across domains in PD psychosis might be leading to more severe cognitive impairments and then dementia. Our results suggest a parallel observation proposing the inclusion of language-based tests (ie, semantic category-based fluency), along with assessments like the intersecting pentagon,46 for enhanced screening to identify dementia progression in PD, especially in PD psychosis. Lastly, we also observed a significantly worse trajectory of performance in patients with PDnP compared with HC across two cognitive tasks, after adjusting for covariates of interest. Although we had predicted that this would be evident across all cognitive domains, we found that this difference was significant only for SDM (processing speed) and MoCA. This is an expected result as healthy individuals generally report less severe cognitive decline compared with individuals with neurodegenerative diseases.62
Limitations, strengths and future research
This study has limitations, which must be balanced against it being the largest and most comprehensive longitudinal sample to date, accounting analytically for the correlated nature of the repeated measurements in each individual (summarised here and discussed in greater detail in online supplemental material 4). In brief, limitations relate to operationalisation of the definition of PD psychosis that may have lacked specificity and reduced sensitivity to detect group differences, and not examining cognitive trajectories from baseline to immediately before the onset of psychotic symptoms, potentially confounding the magnitude of cognitive decline in PD psychosis. Although sensitivity analyses excluding patients on antipsychotics suggest otherwise, inclusion of patients on antipsychotic medications known to affect cognitive performance38 39 may have confounded our results. Nevertheless, we were able to detect significant differences between PDP and PDnP in the longitudinal course of cognitive task performance. Future studies need to address these limitations (see online supplemental material 4), and consider exploring the association between genetic influences in PD and the presence of psychosis symptoms in light of evidence of their role in cognitive decline in PD such as in glucocerebrosidase-related PD.63–65
In conclusion, we examined the trajectory of cognitive performance in patients with PD psychosis and compared it with that of patients without PD psychosis. Patients with PD psychosis showed severe deterioration across most domains, especially semantic aspects of language and processing speed, independent of sociodemographics, neuropsychiatric symptoms and motor symptom severity. These findings indicate a potential role of semantic processing in PD psychosis, which should be further examined. Semantic and other language impairments could pave the way to a deeper understanding of psychosis in PD as well as lead to more targeted treatment.
Data availability statement
Data are available on reasonable request. The data used in this analysis are openly available from the PPMI study. Data used in the preparation of this article were obtained (on 1 February 2023) from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/access-dataspecimens/download-data), RRID:SCR 006431. For up-to-date information on the study, visit www.ppmi-info.org.
Ethics statements
Patient consent for publication
Ethics approval
This study was approved by the Institutional Review Board (IRB) WCG in the USA, and subsequently by the local Research and Development office and Ethics Committee at each site. More information on this can be found on https://www.ppmi-info.org/. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
Data used in this work were obtained from the Parkinson’s Progression Markers Initiative database (http://www.ppmi-info.org/). PPMI—a public-private partnership—is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including 4D Pharma, AbbVie, AcureX, Allergan, Amathus Therapeutics, Aligning Science Across Parkinson’s, AskBio, Avid Radiopharmaceuticals, BIAL, Biogen, Biohaven, BioLegend, BlueRock Therapeutics, Bristol-Myers Squibb, Calico Labs, Celgene, Cerevel Therapeutics, Coave Therapeutics, DaCapo Brainscience, Denali, Edmond J. Safra Foundation, Eli Lilly, Gain Therapeutics, GE HealthCare, Genentech, GSK, Golub Capital, Handl Therapeutics, Insitro, Janssen Neuroscience, Lundbeck, Merck, Meso Scale Discovery, Mission Therapeutics, Neurocrine Biosciences, Pfizer, Piramal, Prevail Therapeutics, Roche, Sanofi, Servier, Sun Pharma Advanced Research Company, Takeda, Teva, UCB, Vanqua Bio, Verily, Voyager Therapeutics, the Weston Family Foundation and Yumanity Therapeutics. For up-to-date information on the study, visit www.ppmi-info.org.