WHAT IS ALREADY KNOWN ON THIS TOPIC
WHAT THIS STUDY ADDS
-
Women with personal and family psychiatric history combined face the highest absolute risk of mild/moderate (11.7%) and severe (2.2%) postpartum depression. Alone, personal history was the most potent risk factor and dose–response relationship based on severity of personal and family history exists.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Background
Depression is the most common psychiatric disorder in the postpartum period, with an estimated pooled prevalence of 10–15% in high-income countries.1 The ramifications of untreated postpartum depression (PPD) are profound.2 Its impact extends beyond the individual to affect the entire family, including increased risks for developmental problems in the offspring, attachment problems between mother and child and family dissolution.2 At a societal level, PPD negatively influences health expenditures and results in productivity loss.3 Thus, identification of high-risk women is essential to assist prevention or early treatment and thereby mitigate both personal and societal consequences.4
Identifying high-risk women remains complex, as reflected in limited and inadequate clinical guidelines.5 Reasons for this are most likely multiple but could be explained by the fact that episodes of PPD can be influenced by a multitude of risk factors as evidenced by umbrella reviews.6 7 However, these factors rarely operate in isolation; rather, they are intertwined. Despite this complexity, current literature largely focuses on isolated risk factors and fails to comprehensively analyse the combined effects of coexisting risk factors. Therefore, there is a pressing need for research that elucidates the interplay between these factors and their collective contribution to PPD risk. Such understanding is pivotal for refining risk assessment strategies and developing personalised and targeted interventions to support high-risk women effectively.
Several PPD risk factors have been identified, with personal history of psychiatric disorders (PH) being the most significant.8 A nationwide American cohort study found that psychiatric diagnoses as major depressive disorder (MDD), anxiety, panic disorders, bipolar disorder (BP), obsessive compulsive disorder (OCD), posttraumatic stress disorder (PTSD) and eating disorders each independently increase risk of PPD.9 Further, a Danish register-based study documented a recurrence risk of 25.5% at second delivery for women who experienced a mental disorder after their first delivery, and 56.8% at third delivery for women with mental disorders in relation to both previous deliveries.10 Likewise, family history of psychiatric disorders (FH) has been shown to be an important predictor in identifying high-risk women.11 Our recent systematic review and meta-analysis estimated a nearly doubled PPD risk among women with FH compared with those without.12 Moreover, a genetic study reported higher heritability of PPD than of general depression, highlighting a genetic link in PPD aetiology.13 PH and FH often co-occur due to the substantial heritability of psychiatric disorders and shared environment among family members.14 However, no studies have yet examined the association of both PH and FH with PPD risk in detail. Thus, the magnitude of the combined contribution to PPD risk remains to be elucidated. Jointly, this impedes strategic efforts for early identification of high-risk women based on two key risk factors: PH and FH.
Reporting of risk in PPD studies is key in supporting both dissemination but also intervention and treatment planning. Epidemiological studies often report relative risk (RR) estimates, which describe the risk of an outcome when comparing two groups with and without the exposure of interest. However, reporting only RR estimates without also reporting the underlying absolute risk makes judgement of the actual clinical relevance difficult. Presentation of absolute risk estimates aids understanding of the importance of the risk factor and provides essential information for gauging an individual’s actual probability of developing the outcome.
Adding to this, it is crucial to recognise that episodes of PPD vary in severity, ranging from mild and moderate episodes to severe ones requiring treatment.8 This variability underscores the complexity of identifying and understanding PPD. Though, most studies have focused on either end of the PPD severity spectrum. To our knowledge, no study has yet included the entire range of PPDs while focusing on both relative and absolute risks for a more complete overview of PPD risks, while at the same time also focusing on combinations of several PPD risk factors.
Objective
In our study, we aimed to examine the absolute risk and RR of developing mild/moderate and severe PPD by two key risk factors, PH and FH, and to report these risk estimates both independently and in combination. We hypothesise that women having both PH and FH will constitute a clinically relevant high-risk group, and that the combined impact of these two risk factors will be greater than the sum of their individual effects.
Methods
Study design, data sources and participants
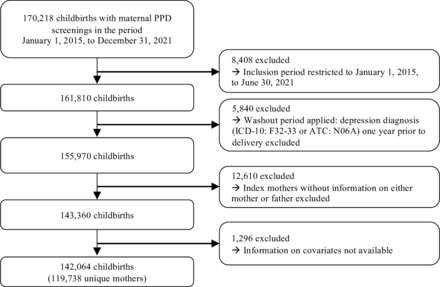
We conducted a cohort study by merging data from the Danish population-wide registers with the HOPE cohort, which encompasses nationwide maternal PPD screenings conducted within the initial 12 weeks post partum from 1 January 2015 to 31 December 2021.15 Initially, the dataset comprised 170 218 childbirths with maternal PPD screenings conducted by healthcare nurses. We included childbirths within the HOPE cohort where the mothers were screened for PPD between 1 January 2015 and 30 June 2021, to allow for 6 months of follow-up, resulting in a sample of 161 810 childbirths. To ensure a distinct separation between the exposure, PH, and outcome, PPD, we implemented a washout period. Washout was defined as 1 year prior to delivery, where we excluded all mothers who experienced a depressive episode (hospital contact with the diagnoses International Classification of Diseases, version 10 (ICD-10) codes: F32–33 or filling prescription of Anatomical Therapeutic Chemical (ATC): N06A), leaving 155 970 childbirths. We further excluded 12 610 childbirths where the index mother cannot be linked to either of her parents in the Danish registers. Finally, we excluded 1296 childbirths where information on covariates was not available. Consequently, this yielded a final sample of 142 064 childbirths of 119 738 unique mothers (figure 1).


Flow chart of the study population. ATC, Anatomical Therapeutic Chemical; ICD-10, International Classification of Diseases, version 10; PPD, postpartum depression.
The HOPE cohort is a large prospective cohort holding nationwide detailed information on PPD symptoms and recorded diagnoses.15 Information on PPD symptoms has been obtained from healthcare nurse records in 76% of Danish municipalities using the Edinburgh Postnatal Depression Scale (EPDS).16 These EPDS screenings have been linked to nationwide register information on recorded diagnoses (filled antidepressant prescriptions and hospital contacts with depression diagnosis) from the National Prescription Register and the Psychiatric Central Research Register.17 18 Furthermore, the HOPE cohort includes important sociodemographic features and PPD risk factors from the National Prescription Register, the Psychiatric Central Research Register, the National Patient Register, the Medical Birth Register and Statistics Denmark’s registers on socioeconomic position.17–20 An evaluation of the HOPE cohort’s representativeness on selected perinatal characteristics demonstrated that the cohort is representative of the background population, ensuring generalisability of findings derived from this cohort. Elaboration of data collection, cohort characteristics and evaluation of selection into the cohort can be found elsewhere.15
Exposures: personal history (PH) and family history (FH)
The exposures were defined as either a prescription filled for psychotropic medication (ATC codes: N05–06) or a psychiatric hospital main or secondary diagnosis (ICD-10: F00–99) from 1995 until the index mother gave birth in the period between 2015 and 2021. This ensured a minimum of 20 years of exposure time. Information was obtained from the National Prescription Register and the Psychiatric Central Research Register.17 18 This definition applied to the index mother (PH) and her parents (FH). Based on this, four exposure groups were defined: PHno & FHno, PHno & FHyes, PHyes & FHno and PHyes & FHyes.
PH and FH were further categorised according to severity of hospital diagnoses based on the in-built hierarchy in the ICD-10 classification: severe (ICD-10: F00–F31), less severe (ICD-10: F32–F99 or ATC: N05–06) and none. If a woman had multiple diagnoses, the most severe according to the ICD-10 hierarchy was used for categorisation. The ICD-10 codes F00–F31 include severe disorders such as schizophrenia and BP, while F32–F99 includes, for example, anxiety and neurodevelopmental disorders.
Outcome: PPD
PPD was delineated into two outcome types: mild/moderate and severe PPD. Mild/moderate PPD were defined as an EPDS score of 11 or above, according to the Danish validation, within the initial 12 weeks post partum.16 Severe PPD was defined as either a filled antidepressant prescription (ATC code: N06A) obtained from the National Prescription Register or a psychiatric hospital contact with main or secondary depression diagnosis (ICD-10: F32–33) obtained from the Psychiatric Central Research Register within the initial 6 months post partum.17 18
Covariates
Maternal age at delivery (continuous) and calendar year of delivery (years 2014 through 2021) were extracted from the Civil Registration System.21 Parity information was obtained from the Medical Birth Register and categorised as 1, 2 and 3+.20 Information on educational attainment was obtained from Statistics Denmark’s Register of Education and categorised according to the International Classification of Education as mandatory, short, medium and high.22
Statistical methods
Descriptive statistics were calculated, encompassing the distribution of various sociodemographic characteristics according to exposure status in the study population.
We estimated absolute risk of PPD according to exposure groups along with 95% Wald CIs. Absolute risk estimates were estimated for both outcome measures: mild/moderate and severe PPD.
Poisson regression analysis was employed to calculate crude and adjusted RRs with 95% CIs for the association between exposure status and PPD. All analyses were conducted with mild/moderate and severe PPD separately. To address dependency between observations in the study population arising from multiple births by the same mother, robust SEs were applied. The adjusted models controlled for maternal age, parity, highest attained maternal education and calendar year of delivery. Given the non-linear relationship of maternal age on the log-odds scale, it was included as a continuous variable modelled by a cubic spline with five-knot points.
Two sensitivity analyses were conducted: (a) The sample was restricted to primiparous women to further account for dependency between observations and to address the impact of selection into motherhood on the results.10 (b) Severe PPD was defined within 12 weeks post partum to maintain consistency in the timeframe for the two outcome measures.
Statistical analyses were performed using Stata V.15. The study was reported following Strengthening the Reporting of Observational Studies in Epidemiology guideline.
Findings
Among the 142 064 included childbirths, 33 218 (23.4%) had neither PH nor FH, 67 382 (47.4%) had FH but not PH, 8535 (6.0%) had PH but not FH and 32 929 (23.2%) had both PH and FH. See table 1 for details.
Characteristics of the study population separated on personal and familial history of psychiatric disorders
The four exposure groups were comparable in terms of maternal age at delivery and calendar year of delivery. A tendency towards lower educational levels was observed among the groups having PH and the combination of PH and FH compared with only having FH or none of the two. For parity status, slight differences were observed with larger proportions of women having three or more children in the group having both PH and FH (14.6%), and vice versa, with larger proportions of women without PH and FH in the group having only one child (9.8%).
In tables 2 and 3, absolute risk and RR of mild/moderate and severe PPD, respectively, in relation to exposure status with 95% CI, are presented. Absolute risk and RR of mild/moderate and severe PPD increased with increasing severity of PH and FH. The highest risk of mild/moderate PPD was observed among women having both exposures with an absolute risk of 11.7% (95% CI 11.5%; 11.8%) and an adjusted RR of 2.35 (95% CI 2.22; 2.49). The same pattern was seen for severe PPD with an absolute risk of 2.2% (95% CI 2.1%; 2.3%) and an adjusted RR of 7.40 (95% CI 5.93; 9.25). Women having only PH without FH also had markedly increased risk of developing mild/moderate (absolute risk: 9.8% (95% CI 9.7%; 10.0%); adjusted RR: 1.99 (95% CI 1.84; 2.16)) and severe (absolute risk: 1.7% (95% CI 1.6%; 1.7%); adjusted RR: 5.90 (95% CI 4.53; 7.69)) PPD. Women having only FH had as well increased risk of mild/moderate (absolute risk: 5.8% (95% CI 5.7%; 5.9%); adjusted RR: 1.18 (95% CI 1.12; 1.25)) and severe (absolute risk: 0.5% (95% CI 0.4%; 0.5%); adjusted RR: 1.70 (95% CI 1.35; 2.15)) PPD.
Absolute risk and relative risk of mild/moderate PPD
Absolute risk and relative risk of severe PPD
The sensitivity analyses of primiparous women and severe PPD restricted to 12 weeks post partum revealed a consistent pattern mirroring that observed in the primary analyses (online supplemental eTables 1–3).
Discussion
To provide a more nuanced and complete overview, we in this study examined the association between personal and family history of psychiatric disorders and RR and absolute risk of developing mild/moderate and severe PPD. Our findings revealed a key link between prior personal and family mental health disorders—such as schizophrenia, BP and MDD—and an elevated risk of PPD. While the risk of PPD remained increased with FH alone, it was notably lower than the risk observed for PH alone. In absolute terms, 11.7% (95% CI 11.5%; 11.8%) and 2.2% (95% CI 2.1%; 2.3%) of mothers having both PH and FH developed mild/moderate or severe PPD. In relative measures, this corresponded to adjusted RRs of 2.35 (95% CI 2.22; 2.49) and 7.40 (95% CI 5.93; 9.25).
Our results underscore several points that can feed into next steps towards personalised prediction of PPD risk. Having a PH is the most potent risk factor for developing PPD. This finding aligns with comprehensive umbrella reviews, risk prediction models and several systematic reviews consistently identifying it as a critical risk factor.6 7 23 Importantly, our research adds to this body of evidence by revealing a more nuanced perspective showing a dose–response relationship based on the severity of PH, which is important to recognise when identifying high-risk women in clinical practice. We observed a substantial risk for PPD among women having severe PH according to the ICD-10 hierarchy, such as substance use disorders, schizophrenia and BP, while a lower increased risk of PPD was observed among women with less severe PH such as MDD, anxiety, eating and personality disorders. However, it is important to acknowledge that all women with PH do not develop PPD, as evidenced by tables 2 and 3.
Another main finding is that FH is a risk factor for PPD, although to a much lesser extent than PH. The risk of severe PPD when having a parent with past psychiatric disorders aligns with our recent systematic review and meta-analysis reporting an almost doubled risk of PPD in individuals with an FH (adjusted RR for less severe FH, 1.70 (95% CI 1.34; 2.15) and adjusted RR for severe FH, 1.79 (95% CI 1.18; 2.73)).12 Severity of FH, unlike severity of PH, did not have a consistent effect on PPD risk. Several other PPD risk factors have been found to exert risk in the same range as FH including gestational diabetes (RR 1.59 (95% CI 1.22; 2.07)), preterm birth (RR 1.79 (95% CI 1.46; 2.21)) and emergency caesarean section (OR 1.53 (95% CI 1.22; 1.91)).24–26 Thus, it is clear that FH is one among several PPD risk factors increasing the risk of PPD in roughly the same magnitude and range, although direct comparisons between studies are challenging due to differences in study population, design and available data sources.
Despite a wealth of identified PPD risk factors, it remains difficult to accurately predict who will develop PPD due to the intricate nature of its underlying disease mechanisms.6 7 Our study adds to this line of work by shedding light on the combination of two well-known risk factors, as well as addressing a critical gap in existing evidence by quantifying in absolute terms how many women develop or do not develop PPD if they have the outlined risk factors of interest in combination. Guidelines for identification of high-risk women are lacking and of worryingly low quality.5 Building on this, recommendations from the US Preventive Service Task Force Recommendation Statements highlight that women at increased risk should be referred to counselling interventions. However, this is recommended while also emphasising that evidence-based approaches to identify high-risk women are lacking.27 Clinical guidelines are important for clinical practice, since PPD is underdiagnosed and undertreated.28 Importantly, such guidelines are still oversimplified since they do not account for differences in risk impeding personalised interventions and treatment. Based on current evidence, we advocate for development of future clinical guidelines focusing on more nuanced and personalised stratification of PPD risk profiles. This could entail evaluation of the presence of combined specified PPD risk factors among pregnant or postpartum women. This would be in line with recommendations from the US Preventive Service Task Force Recommendation Statements, suggesting examination of specified risk factors.27 Moreover, in addition to personal and familial psychiatric history, future studies could consider examining the impact of the maternal partner’s psychiatric history to create a more comprehensive understanding of the familial environment and potential PPD risk factors.29
Strengths and limitations
We used the HOPE cohort, a highly unique and unprecedented dataset which has been shown to be representative of the Danish background population with no indication of selection bias.15 To our knowledge, this dataset represents the largest cohort with both EPDS and register data. We identified PPD with varying severity using multiple measures, including EPDS questionnaires, prescription medication and treatment at specialised psychiatric treatment facilities. We showed an increased risk of mild/moderate and severe PPD, although with difference in magnitude indicating a larger contribution of both PH and FH to RR among women having severe PPD (table 3) than among women having mild/moderate PPD (table 2). A major strength of using registers and records for case identification is the elimination of the risk of recall bias.
Our study also has limitations. We excluded 12 610 (8%) mothers who did not have register information on either mother or father of index individuals, thus information on FH was not accessible. This group of women was fully characterised by not being Danish-born women, with 99% of the women being of non-Danish origin (results not shown). Thus, our findings may not generalise to non-Danish-born populations. Moreover, we categorised severe PPD as either a hospital depression diagnosis (including both inpatient and outpatient diagnoses) or a filled antidepressant prescription, which served as a proxy for diagnoses made by general practitioners. We did, however, not have information on diagnoses made specifically by general practitioners. Note, prior research from our group has demonstrated that antidepressant prescriptions are reliable proxy measures for a depression diagnosis.30
Clinical implications
In this population-based cohort study, we provide novel nuances on the association between combination of key PPD risk factors and absolute risk and RR of different severity levels of PPD. We found an association between prior personal and family history of psychiatric disorders, both alone and in combination, and mild/moderate PPD identified through questionnaire data and severe PPD identified as records of psychiatric diagnosis or prescription drug use. The risk of PPD was, not surprisingly, elevated most with PH and FH in combination, and thus should be evaluated when trying to identify high-risk women. Alone, personal history was the most potent risk factor, while family history was a lesser risk factor. Precise identification of high-risk women remains worryingly difficult. Our findings could assist in more detailed risk assessment strategies and highlight the need for further research examining the interplay between coexisting risk factors and their contribution to PPD risk. While current guidelines for identification of high-risk women often recommend universal screening, we advocate for a more nuanced and personalised risk stratification in future clinical guidelines.
Data availability statement
No data are available. According to Danish data protection laws, person-level data will not be available for the public. Data are stored on a secure platform on Statistics Denmark available only to authorised personnel.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the Danish Data Protection Agency (through local registration at Aarhus University, journal number 2016-051-000001, serial number 2304). Data have been anonymised, and all analyses were conducted on a secure platform on Statistics Denmark. Informed consent is not required for this study, according to Danish law.